Dental zirconia and keys for clinical success
Explaining how one of the most popular indirect restorative material is formed and functions.
Dentists have increasingly requested zirconia as an alternative to porcelain-fused-to-metal (PFM) restorations and more recently to glass ceramic restorations as well.
For more than 15 years, zirconia has been used for fabricating restoration frameworks based on the material’s versatility in mechanical and physical properties, which has allowed clinicians and laboratory technicians to use it for various clinical indications.
The first zirconia restorative materials on the dental market were 3Y-TZP powders. Although these materials had high mechanical properties, they were dense and opaque, falling short of meeting dentists’ requirements for esthetics, which were equally important to strength considerations. Since then, the number and compositions on dental zirconia materials have grown rapidly.
With recent advancements, a variety of zirconia materials (4Y-TZP, 5Y-TZP) have become available to meet dentists’ different functional and esthetic demands. Differentiated by a number of factors, including composition, mechanical, and optical properties, today’s new zirconia materials offer dentists and laboratories solutions that can be milled to full contour and demonstrate acceptable esthetics and translucency suitable for clinical situations where high mechanical stability, thin restoration walls and natural esthetics are essential.
Trending article: Why dentists should take a fresh look at new restorative innovations
What is dental zirconia?
Fig. 1
Dental zirconia (ZrO2) is the oxide version of zirconium (Zr). Zirconium occurs in nature only as a mineral-mostly as zircon (ZrSiO4)-and is a soft, ductile, shiny-silvery metal, optically similar to aluminum foil.1,3
To produce dental zirconia, zirconis purified via complex production and purification processes and converted into synthetic zirconium precursors, which are finally transformed into ZrO2 through thermal and mechanical processes. These are the only synthetic powder components used to make dental zirconia.1-3
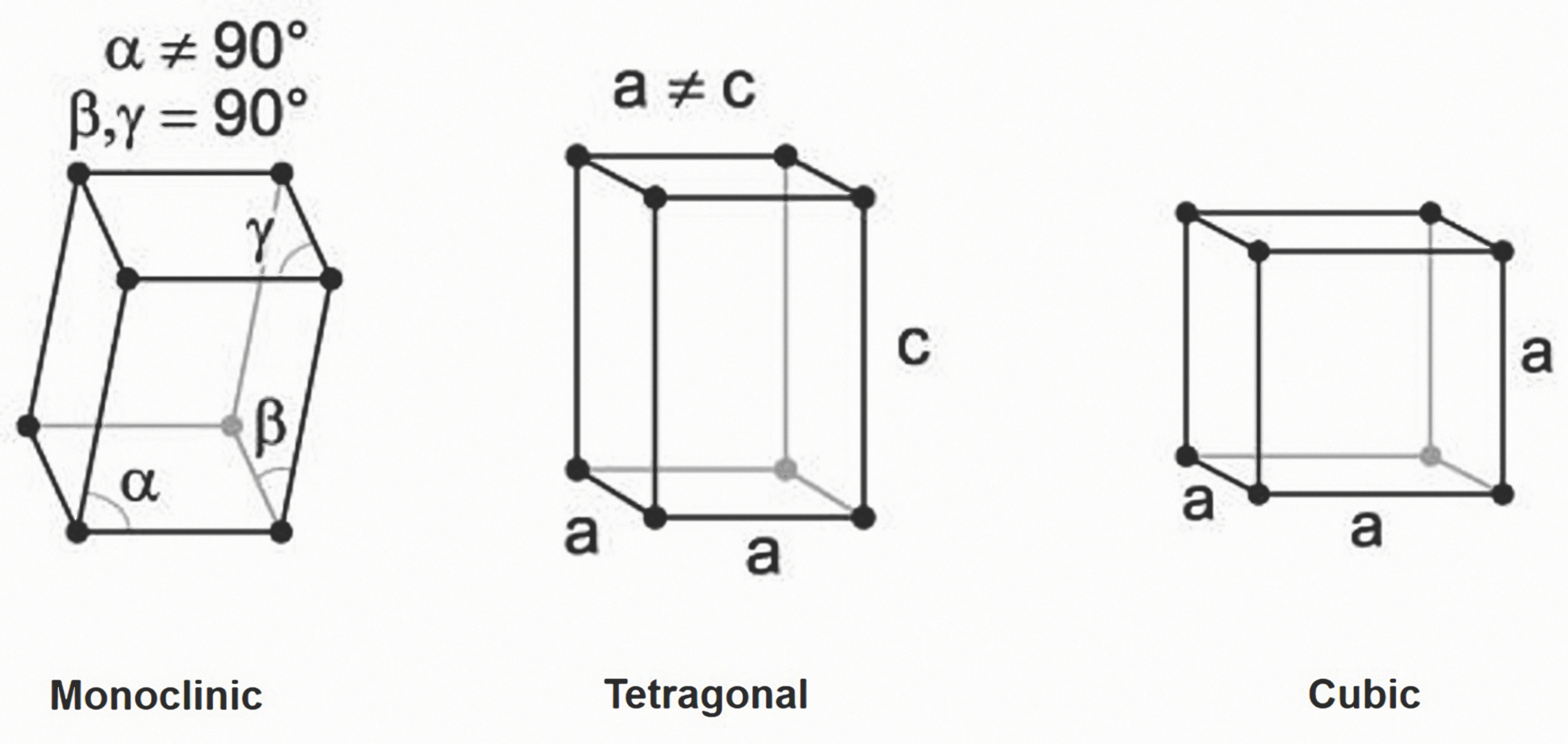
Zirconia is polymorphic ceramic; depending on temperature and pressure, the same elements of the material exist in three different crystal structures (i.e., monoclinic (m); tetragonal (t); and cubic (c)) (Fig. 1). Pure monoclinic zirconia, the most stable phase, is present at room temperature. At about 1,170° C, the monoclinic phase transforms into the tetragonal phase, with an approximately 4 to 5 percent volume shrinkage. At about 2,370° C, the tetragonal phase then converts into the cubic phase. These transformations occur within a temperature range (rather than at a specific temperature) and involve movement of atoms within the crystal structure.
The tetragonal and cubic phases of zirconia can be made stable at room temperature by incorporating additional components (dopants), such as yttrium oxide (Y2O3), calcium oxide (CaO) or magnesium oxide (MgO) into the ZrO2 crystal structure to form partially or fully stabilized zirconia.1-3
Without the addition of these components, tetragonal converts back into a monoclinic below 950° C and, hence, cannot be used clinically. Low amounts of these dopants lead to partially stabilized zirconia, with mainly metastable tetragonal and cubic phases.1-3
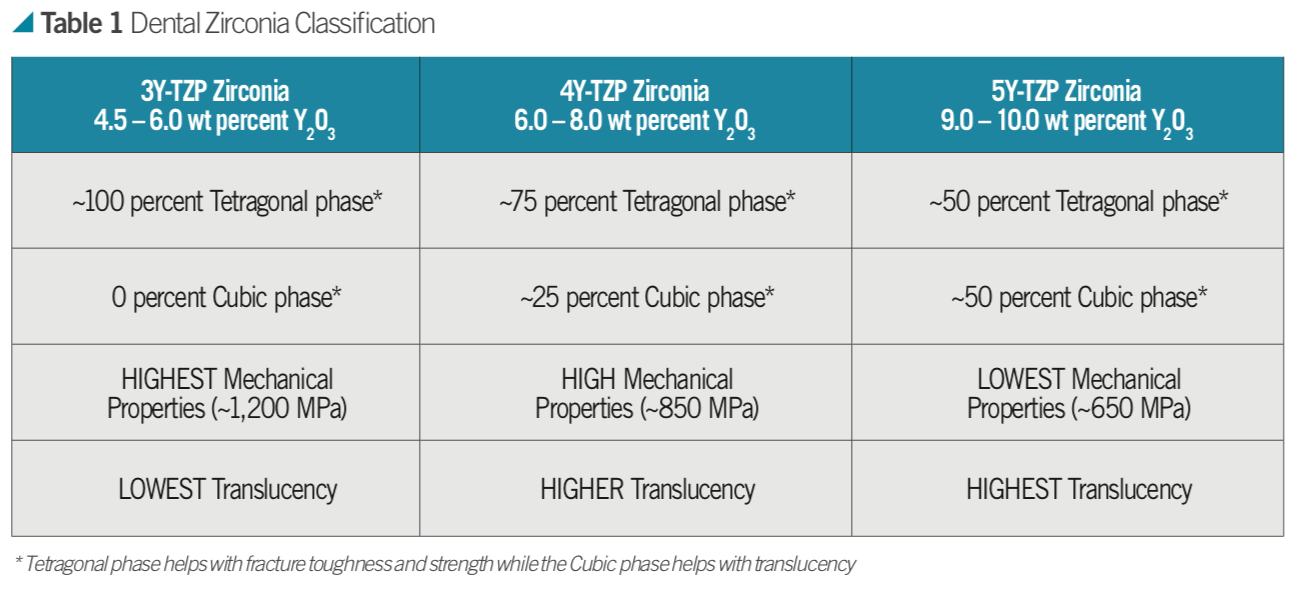
For example, how much dopant in molar concentration is used in a zirconia is abbreviated as 3Y-TZP for 3 mol percent Y2O3; 4Y-TZP as 4 mol percent Y2O3; or 5Y-TZP as 5 mol percent Y2O3.
When approximately 4.5 to 6 weight percent (3 mol percent or 3Y-TZP) yttria is added to a structure, a 100 percent tetragonal phase (traditional dental zirconia) can be produced at room temperature. When approximately nine to 10 weight percent (5 mol percent or 5Y-TZP) yttria is added, a structure of 50 percent tetragonal/50 percent cubic phase (known as cubic or HT zirconia) can be produced at room temperature. When these powders are mixed, an approximately 6.5 to 8 weight percent yttria containing zirconia can be produced (4 mol percent or 4Y-TZP) giving a microstructure of 75 percent tetragonal and 25 percent cubic (Table 1).
Trending article: How to break out of your restorative rut
The composition of zirconia material defines its mechanical and physical properties and hence clinical indications. The biaxial flexural strength of zirconia materials ranges from 650 MPa (5Y-TZP) to 1,200 MPa (3Y- TZP). The higher the value, the stronger the material.
In addition, the presence of poly-morphic phases in zirconia materials provides a phenomenon known as phase transformation toughening. It causes the tetragonal crystals to change to monoclinic when a crack is introduced. The monoclinic phase has a greater volume. This stops the crack from traveling through the material, basically pinching the crack shut and, hence, further increases resistance to fracture (Fig. 2).
No phase transformation toughening can be observed in 5Y-TZP materials. Lastly, the translucency of 3Y-TZP is comparatively lower than 4Y-TZP and 5Y-TZP which is the most translucent, resulting in a decision-making tree for clinical indications and cementation procedures.
Related reading: What makes a zirconia stand out?
Fig. 2: A visual representation of the phase transformation toughening phenomenon that allows zirconia to seal cracks on its own.

Fig. 2: A visual representation of the phase transformation toughening phenomenon that allows zirconia to seal cracks on its own.
Ensuring success with different zirconias
As discussed earlier, the obvious disadvantage of new higher translucency and more esthetic ZrO2 materials is a reduction in the mechanical properties (e.g., lower fracture toughness, lower strength). There is a growing interest in using zirconia for fabricating monolithic, full-contour restorations-particularly different generations that demonstrate new levels of optical and mechanical properties to meet dentists’ demands. The composition, mechanical properties, optical characteristics and processing of these new zirconias are different from previous generations of the high-strength material.4,5
Trending article: Tips and tricks for creating long-lasting restorations
Fig. 3: Featuring a unique gradient technology, IPS e.max ZirCAD Prime is the newest generation of zirconia restorative materials.
Currently, newer generation cubic - 5Y-TZP (e.g., CubeX2) or hybrid - 4Y-TZP (e.g., IPS e.max® ZirCAD MT) zirconia materials are limited to single-unit restorations, or to three unit bridges. These zirconias exhibit improved translucency for esthetic full-contour (i.e., monolithic) restorations, but they demonstrate lower mechanical proper- ties and a reduction in strength and fracture toughness compared to some other restorative materials.6,7 This may limit their use to certain indications, wall thicknesses and connector dimensions.
The 3Y-TZP zirconia materials (e.g., IPS e.max® ZirCAD LT) are indicated for single-unit restorations to multi-unit bridge frameworks with a maximum of two pontics. These materials demonstrate high-strength, excellent mechanical properties and a low risk of temperature degradation; however, they exhibit a slightly lower level of translucency.8
The newest generation of zirconia restorative material (IPS e.max® ZirCAD PRIME) has been introduced with a unique gradient technology (Fig. 3). This technology allows gradation of 3Y-TZP and 5Y-TZP material in one puck, ensuring the strength of 3Y-TZP and esthetics of 5Y-TZP.
Proper preparation
Fig. 4
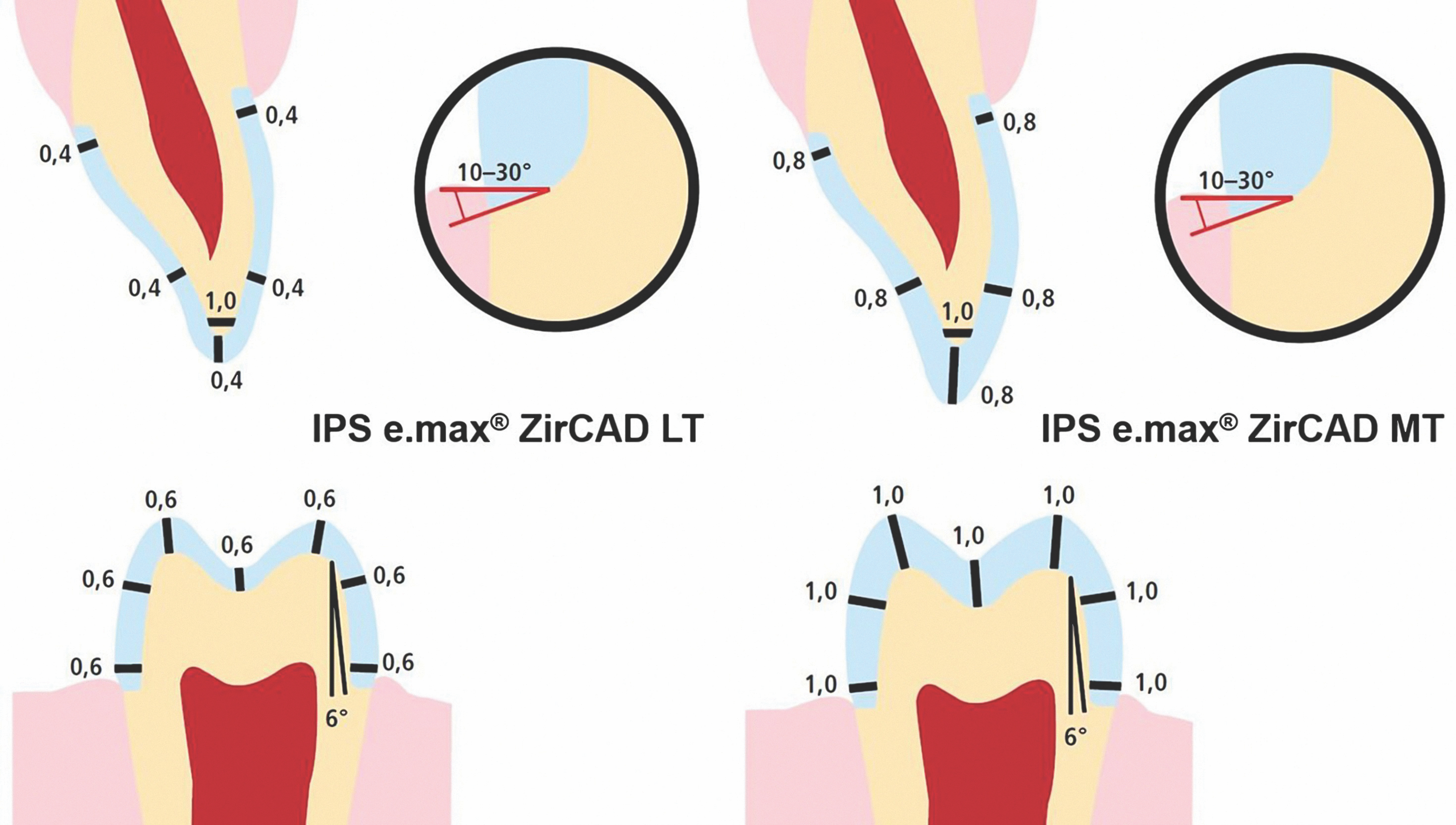
Clinicians should follow tooth preparation guidelines specific to their selected zirconia restorative material. It is also critical for both clinicians and dental laboratory technicians to consider the differences in properties among zirconia materials when selecting the ideal zirconia for a specific clinical indication.
Preparation guidelines for 3Y-TZP zirconia materials range from 1.0 mm to 0.5 mm occlusal and axial reduction, whereas for 4Y-TZP and 5Y-TZP zirconia restorative materials, they range from 1.5 mm to 1.0 mm reduction (Fig. 4). Additionally, the connector dimensions for bridges vary from 12.0 mm2 for 5Y-TZP and 4Y-TZP materials, compared to 7.0 to 9.0 mm2 for 3Y-TZP zirconia materials.
Trending article: 6 materials lab techs need to know about
Optimal adhesive protocols
Further, although there are various cementation options available for use with zirconia restorations (e.g., conventional, self-adhesive, and adhesive cements), it is important to remember that the actual technique-and diligently following its protocol-also influences clinical restorative success. Clinicians often use conventional cements such as resin modified glass ionomers or glass ionomers when placing zirconia restorations, due to their ease of use. However, the limited bonding properties of conventional cements restrict their use in non-retentive tooth preparations.
The common myth is zirconia material cannot be chemically bonded. However, it is well cited in the literature that zirconia restorations can be adhesively cemented if proper steps are followed. To ensure successful cementation, the following critical protocol should be implemented with zirconia restorations. Avoiding any step in the cementation protocol will compromise the clinical outcome.
Fig. 5

- Zirconia restorations cannot be chemically etched. Traditional dental etching procedures are preferential and involve etching away the open glass phase structure in glass-ceramic restoration, such as IPS e.max® lithium disilicate; this leaves the crystals because zirconia has no secondary glass phase. Therefore, sandblasting the intaglio surface of a zirconia restoration using Al2O3 particles (50 μm) at 1 bar pressure-which is usually performed by the dental laboratory-roughens the zirconia surface to increase micro-retention for improved bonding.
- After a try-in of the zirconia restoration in the patient's mouth, it should be cleaned. Zirconia surfaces show a high affinity for phosphate groups, and saliva and other body fluids contain various forms of phosphate (e.g., phospholipids) that may react irreversibly with the restorative surface and compromise bonding.
This also contraindicates the use of phosphoric acid on zirconia restorations. To clean zirconia restorative surfaces after try-in and create an optimum surface for adhesive bonding compared to other cleaning protocols, a unique product (Ivoclean®, Ivoclar Vivadent, Inc.) is indicated (Fig. 5).9,10 - The cementation of zirconia restoration can be performed using an adhesive cement (e.g. Variolink® Esthetic, Multilink® Automix) or a self-adhesive cement (e.g., SpeedCEM® Plus). The cementation protocol includes application of primer on the restoration, followed by the use of cement.
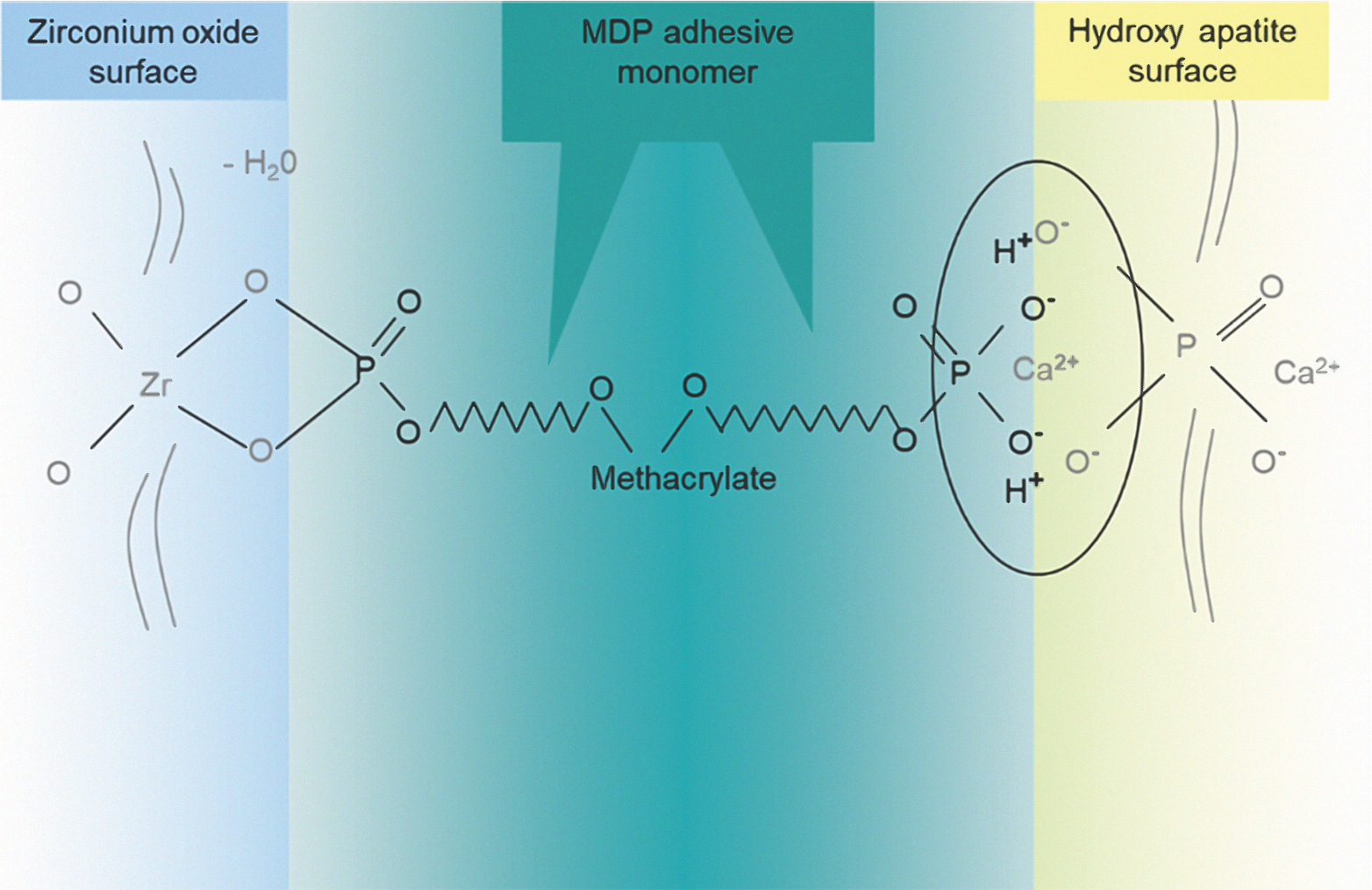
Unlike glass-ceramic bonding, which uses silane bonding, zirconia bonding uses phosphate end groups to bond. The use of primers containing phosphate end groups, or cements containing MDP (10-methacryloyloxydecyl dihydrogen phosphate), is recommended for achieving the best bonds to the tooth structure (Fig. 6). The MDP-containing ceramic primers (e.g. Monobond Plus) should be applied on the restoration followed by extrusion of adhesive resin cement in the restoration.
Because few self-adhesive resin cements (e.g., SpeedCEM® Plus) contain MDP, the application of restorative primer as a separate step can be eliminated - Finally, cement is extruded in the restoration; the doctor should seat it per path of insertion, followed by polymerization of the cement per the manufacturer's recommendation
- Lastly, the translucency of the zirconia restorations depends on the material's composition and thickness, and hence light attenuation through the restoration varies.
Fig. 6
Therefore, it is critical to consider these factors while selecting cement options. For opaque restoration, use of self-cure and dual-cure cements are recommended, and it is extremely important to let the cement set on a self-cure mode before checking occlusion or making occlusal adjustments.
References
- Volpato Maziero CA, D’Altoe Garbelotto LG, Celso Fredel M, Bondioli F. Application of zirconia in dentistry: biological, mechanical and optical considerations. Advances in Ceramics-Electric and Magnetic Ceramics, Bioceramics, Ceramics and Environment. 2011:397-421.
- Chen YW, Moussi J, Drury JL, Wataha JC. Zirconia in biomedical applications. Expert Rev Med Devices. 2016 Oct;13(10):945-963.
- Nielsen RH, Wilfing G. Ullmann. Zirconium and zirconium compounds. Ullmann’s Encyclopedia of Industrial Chemistry. 2010.
- Miyazaki T, Nakamura T, Matsumura H, Ban S, Kobayashi T. Current status of zirconia restoration. J Prosthodont Res. 2013 Oct;57(4):236-61.
- Ramos CM, Cesar PF, Bonafante EA, et al. Fractographic principles applied to Y-TZP mechanical behavior analysis. J Mech Behav Biomed Mater. 2016 Apr;57:215-23.
- Munoz EM, Longhini D, Antonio SG, Adabo GL. The effects of mechanical and hydrothermal aging on microstructure and biaxial flexural strength of an anterior and a posterior monolithic zirconia. J Dent. 2017 Aug;63:94-102.
- Zhang F, Inokoshi M, Batuk M, et al. Strength, toughness and aging stability of highly-translucent Y-TZP ceramics for dental restorations. Dent Mater. 2016 Dec; 32(12):e327-e337.
- Pinto PA, Colas G, Filleter T, DeSouza GM. Surface and mechanical characterization of dental yttria- stabilized tetragonal zirconia polycrystals (3Y-TZP) after different aging processes. Microsc Microanal. 2016 Dec;22(6):1179-88.
- Kim DH, Son JS, Jeong SH, Kim YK, Kim KH, Kwon TY. Efficacy of various cleaning solutions on saliva-contaminated zirconia for improved resin bonding. J Adv Prosthodont. 2015 Apr;7(2):85-92.
- Pathak K, Singhal S, Antonson SA, Antonson DE. Effect of cleaning protocols of saliva-contaminated zirconia-restorations: shear bond strength. J Dent Res. 2015;94 (Spec Iss A):3656.
ACTIVA BioACTIVE Bulk Flow Marks Pulpdent’s First Major Product Release in 4 Years
December 12th 2024Next-generation bulk-fill dental restorative raises the standard of care for bulk-fill procedures by providing natural remineralization support, while also overcoming current bulk-fill limitations.