Bringing early-stage carious lesions to light
Using nanotechnology, GreenMark Biomedical is developing a non-invasive approach to diagnosing and treating active, incipient carious lesions.
The idea of nanodentistry may still seem a little like pure science fiction or a distant vision. In fact, products based on "small scale" targeted technology for diagnosing, treating and preventing caries may well be in your practice in the near future.
Last month I had the opportunity to sit down with Steven and Wendy Bloembergen of GreenMark Biomedical Inc., two pioneers working to make the vision of early detection and noninvasive treatment of caries a near-term reality.
GreenMark is a healthcare startup specializing in targeted biopolymer particle technology. Steven, GreenMark’s founder and CEO, has more than 30 years in the field of biomaterials, while Wendy, a noted nephrologist, serves as GreenMark’s vice president of clinical affairs.
What’s the background behind GreenMark’s core technology?
SB: Since my original Ph.D. research project, I have had a passion for developing sustainable cost-effective alternatives to petroleum-based plastics and other non-biodegradable materials that are used for disposable products and packaging. The core technology, which is based on starch nanoparticles, became the driver behind my first startup company, EcoSynthetix, now a global corporation.
At EcoSynthetix, we were able to replace oil-based chemicals such as styrenebutadiene and harmful formaldehyde-based binders used to produce everyday products like magazines, books, cereal boxes, and wood composite panels. GreenMark was created in 2016 to pursue targeted and noninvasive dental and medical applications with this same technology.
How did you decide to adapt the technology for dental applications?
WB: In early 2000, I helped Steven with the writing of a grant which was funded by NIH to investigate applications of the starch particle technology in healthcare, specifically for drug delivery to fight cancer. This research on a targeted delivery system was set aside for almost a decade. However, it was eventually resurrected as the subject of Ph.D. research by Nathan Jones at the University of Michigan.
The research caught the attention of Brian Clarkson, professor of cariology at the U of M dental school. Brian recognized that in the early stages of tooth decay you develop porosities in the enamel which these sub-micron particles could penetrate. Nathan, now GreenMark’s VP of technology, found a way of targeting these particles to attach to the subsurface of active carious lesions which is truly a breakthrough.
What’s the potential significance of this breakthrough in the detection and treatment of caries?
SB: We know visual and tactile exams using the explorer miss lesions or can cause cavitation. X-rays cannot catch lesions until they’re well advanced-by that time it’s too late for anything but drill and fill. But if you can detect caries in the incipient stage, you have the opportunity to treat noninvasively.
Perhaps even more exciting is the potential of this technology to distinguish between actively progressing and inactive lesions. The small particles only penetrate lesions with surface porosity, which are believed to be those lesions that are active or likely to progress. At present there are no diagnostic techniques or devices capable of differentiating between active and inactive lesions on the market. Dental professionals tell us it’s a guess.
Lastly, we believe the technology will allow dentists to monitor the effect of noninvasive treatments. So, we think this is truly a disruptive technology that has the potential of changing the way things are done in the field of caries management.
So how would a diagnostic application work?
SB: The practice would administer the diagnostic test as part of a routine prophy exam. We have developed a mouth rinse where the targeted starch particles are dispersed at a low concentration in water. The patient rinses first with the diagnostic solution and then with plain water. All that remains after the water rinse are the particles that have penetrated
the tiny porosities in the enamel and attached themselves temporarily to the active subsurface lesions. The particles contain molecules that fluoresce or light up under exposure from a standard blue curing light. Within seconds, the dentist receives a direct visual diagnosis because the illuminated areas indicate areas of porosity.
The starch particles are completely biocompatible and resorbable, making them ideal for use in the oral cavity. They break down in the presence of amylase which is a naturally occurring enzyme in human saliva. So, by the time the patient leaves the office the particles are completely degraded and there is no residual.
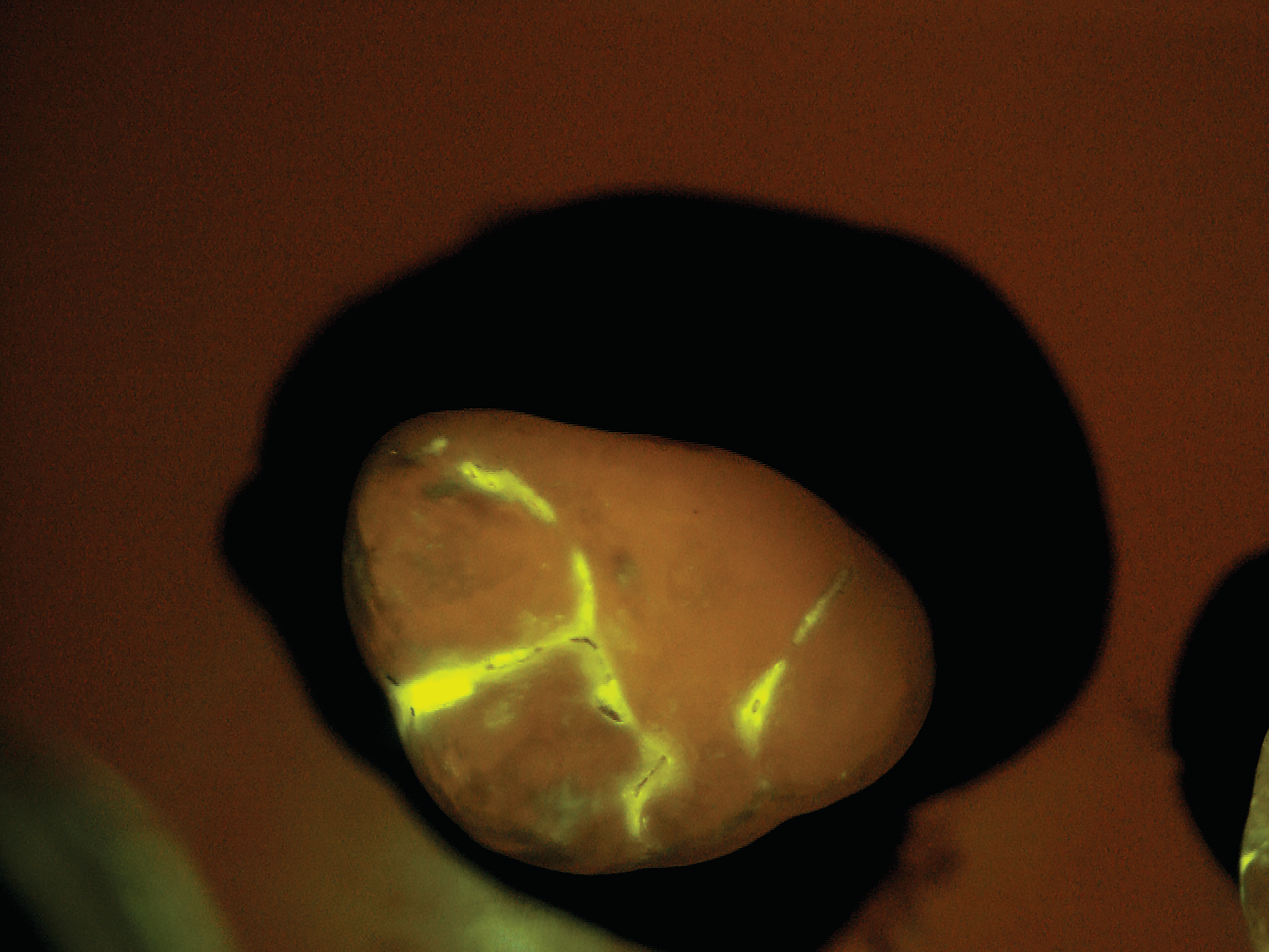
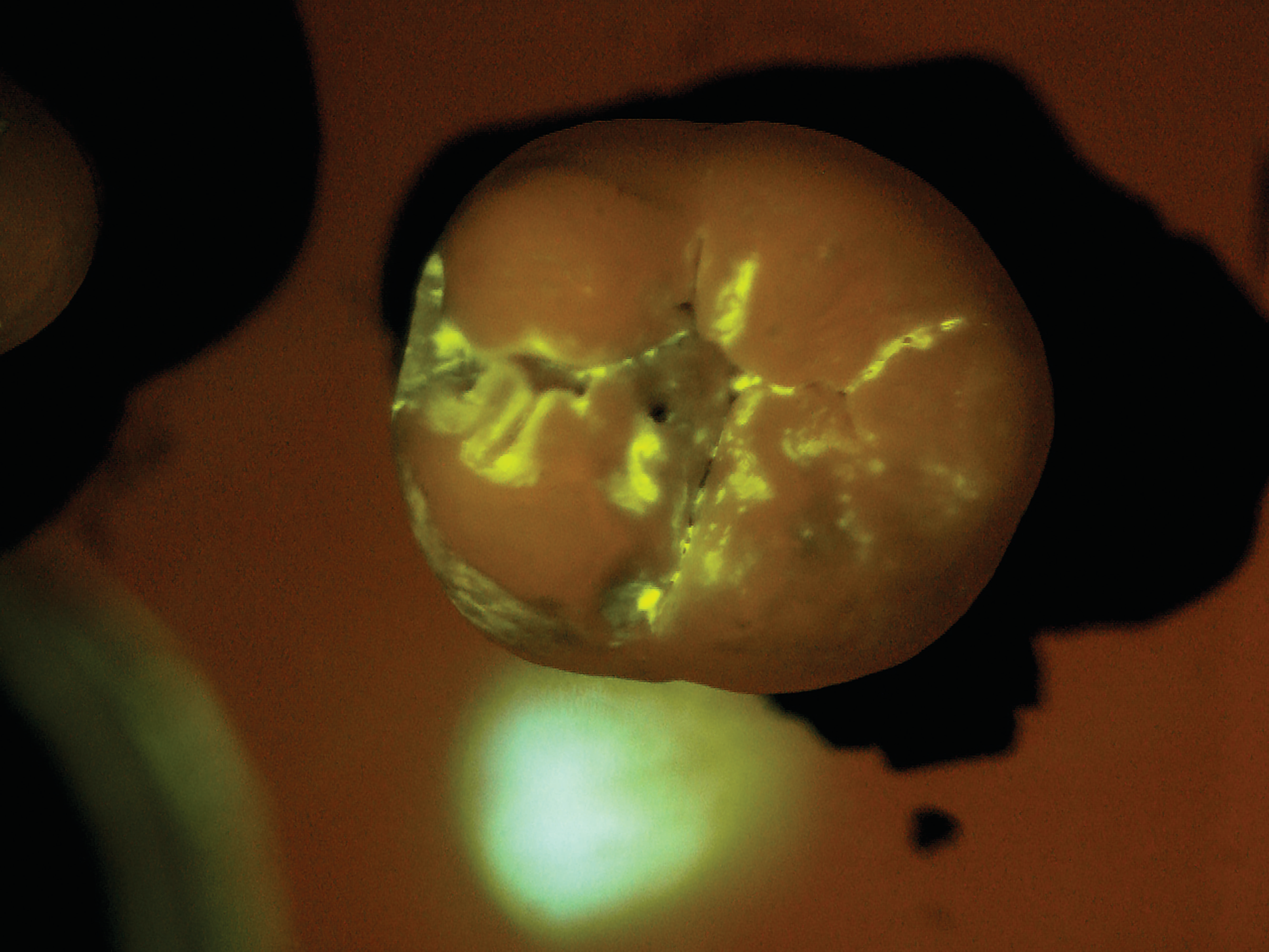
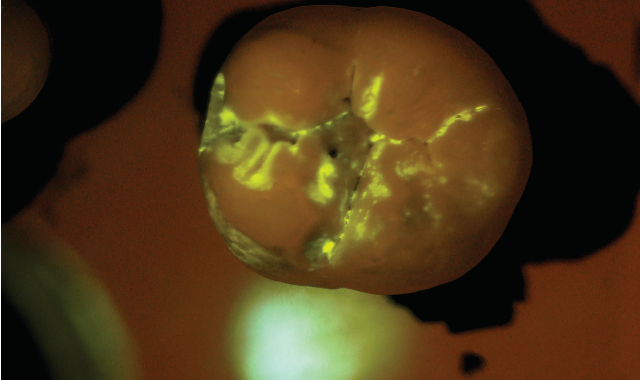
After the patient drinks the mouth rinse containing the targeted starch particles and then rinses with water, particles adhering to enamel porosities fluoresce under a standard blue curing light.
How can the same nanoparticle technology be used in noninvasive treatment?
WB: Calcium and phosphate, the key minerals that are depleted from teeth during the process of decay, can be bonded very effectively inside the small sub-micron starch particles. Since the targeted starch particles adhere to the surface of carious lesions, we have the potential to deliver the building blocks of enamel directly to the site of active disease. The minerals are released when the starch degrades, triggering recrystallization of the tooth subsurface by drawing on large amounts of naturally occurring calcium and phosphate from the saliva in a process known as “crystal nucleation.”
While this application is still in preliminary research, our team has been very excited by the initial test results. In the lab we’ve created artificial lesions on human enamel that look exactly like the white spots that we see in natural lesions. We’ve then treated this enamel with mineral laden particles and over the course of weeks we see a complete return to what looks like natural enamel. Next steps would be to replicate these results in vitro with natural lesions and ultimately in vivo in patients’ mouths.
The implications of these results are very promising. Unlike fluoride treatments that arrest lesions by sealing surface micro channels in the tooth enamel, we see the potential for targeted bio-delivery of minerals which restores the enamel from within, into the depth of the lesion.
How soon could we see products utilizing these technologies available to dental professionals?
WB: The diagnostic application, which we’re calling LumiCare™, is scheduled to be submitted for FDA regulatory review this year. We plan for initial availability in 2020. The noninvasive treatment is still in the research phase, but we’re moving rapidly on that application as well. If readers would like to track our progress, we invite them to visit here.