Biomarker Reader Helps Catch Oral Cancers Earlier
Vigilant Biosciences has developed a method to help dental clinicians accurately detect oral cancers chairside, with the goal of early cancer diagnosis and better survival rates.
Biomarker Reader Helps Catch Oral Cancers Earlier | Image Credit: © Vigilant Biosciences
Head and Neck Cancer Awareness Month occurred in April, and the facts show that oral cancers cannot be ignored. The American Society of Clinical Oncology estimated that in 2023 in the US, 39,290 men and 15,250 women received a diagnosis of oral or oropharyngeal cancer. The 5-year relative survival rate in the US is 68%, but if diagnosed at an early stage, the 5-year relative survival rate jumps to 86%.1
Vigilant Biosciences® has developed an accurate method of detecting oral cancers by identifying biomarkers chairside in the dental office. The BeVigilant™ OraFusion™ System has been granted FDA breakthrough device designation and is expected to receive FDA approval in the coming months. I spoke with Bill Brodie, CEO of Vigilant Biosciences, to find out more.
How did you get involved with Vigilant Biosciences?
Bill Brodie (BB): I’ve been in the dental medical space for 34 years and ran my own dental supply equipment company that was subsequently bought by Benco Dental, which was a small regional company at the time. As vice president of sales, I helped grow the Southeast region for 7 years. After that, I shifted to the medical area, working with cutting-edge artificial intelligence (AI)–driven technologies. The Vigilant Biosciences board saw me as uniquely qualified because of my experience in both medical and dental areas. I’ve been with the company for 5 years.
What is the company trying to accomplish?
BB: The company was originally founded by an attorney whose parents both died of oral cancer. He connected with a biomarker researcher, and they began exploring how salivary biomarkers can predict oral cancer. The first 6 years were spent researching and improving biomarker accuracy. In the past 4 years, after studying thousands of biomarkers, specific biomarkers that focused on the goal of early detection were chosen. In all our studies and trials, at least 50% of the cohort was early stage or precancer stage 0.
Why do dentists need this?
BB: We believe the most benefit is achieved if the dentist can move the decision of whether the patient needs a specialist to when that patient is still sitting in the dental chair. A good example is lichen planus. There is a less than 4% chance of lichen planus turning cancerous. But if it does, it is very aggressive, and the patient can be dead in 18 months.
How does The BeVigilant OraFusion work?
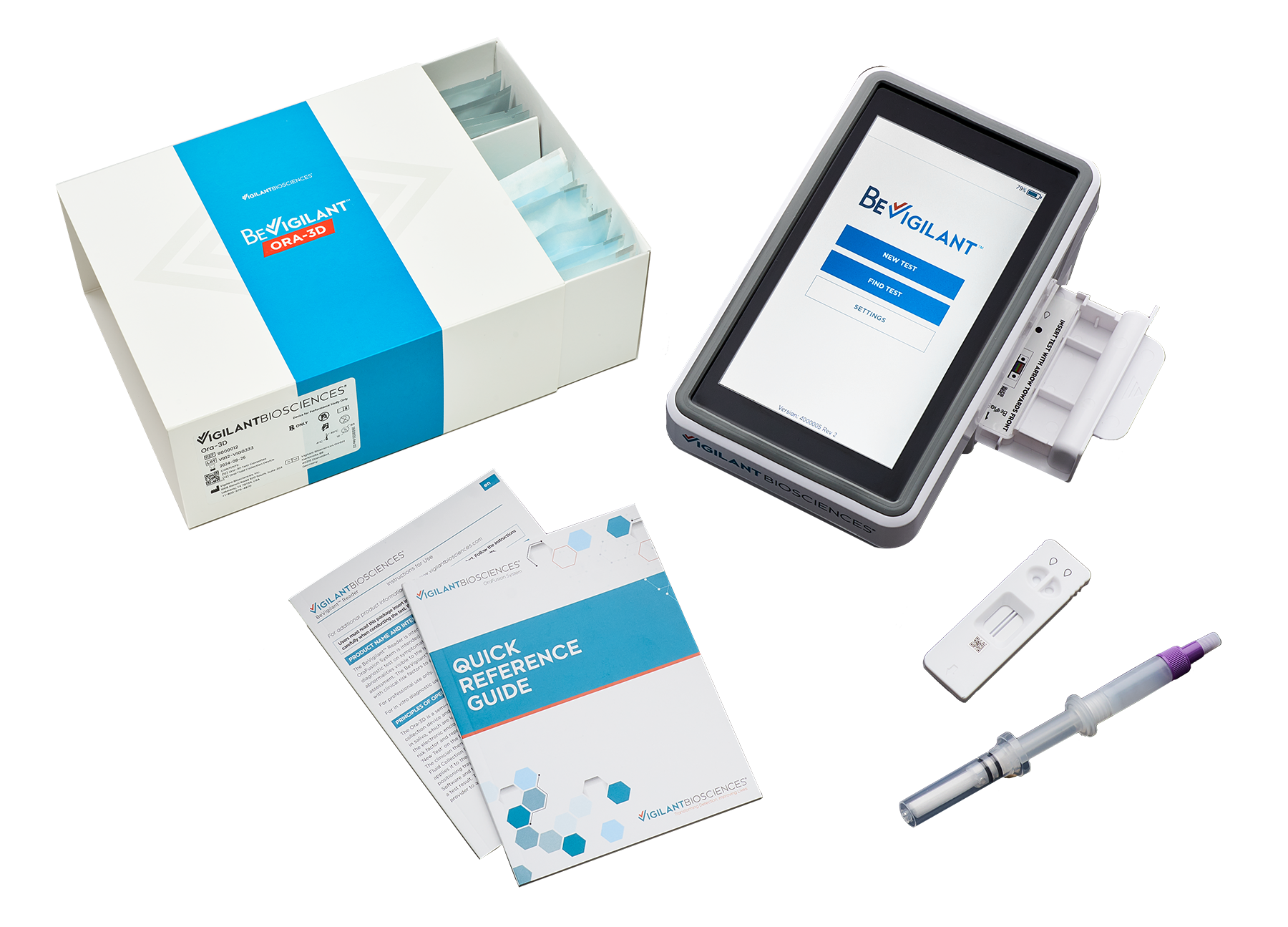
BB: The lateral flow cassette looks similar to home COVID-19 tests. The dentist or hygienist puts 4 drops of saliva on each side. The cassette is then put in the little drawer of the reader, and the software produces a result and gives you a report. The reader has an onboard battery so it can be moved among operatories.
It takes about 10 minutes to get a result. The reader measures the exact concentration of 2 proteomic biomarkers and a control. These proteins become elevated as tissue becomes cancerous.
The control measures the amount of the saliva and the viability of the sample.
The first biomarker, a transmembrane biomarker, detects rapid reproduction of the cells, a hallmark of cancer. This biomarker is shed by the cell membrane as the cells reproduce.
The second biomarker becomes overexpressed as the body attempts to suppress that cellular activity. The relationship between these 2 biomarkers provides information that produces the accuracy.
Combine that with clinical risk factors, such as sex, age, and tobacco and alcohol use, in intelligent machine-learning software, and the ultimate result indicates whether the patient needs to be referred to a specialist. The report can then be emailed.
How do you know which patients to test?
BB: Testing is recommended if a dentist or hygienist sees some abnormality in the patient’s mouth—it could be leukoplakia, lichen planus, red spots, white spots, or anything out of the ordinary. Knowing whether to wait and monitor or refer to a specialist is very hard in the early stage. About 25% to 30% of patients have an oral abnormality. The report shows the relative concentration of the biomarkers, signifying whether the abnormality in the mouth is starting to exhibit cancerous behavior.
What will the machine cost?
BB: Suggested retail for the machine is around $1000 and each cassette is $60 to $70 individually, but they are sold in packs of 12. We are working with reimbursement experts who have suggested an insurance code that is used by light-based devices—CDT 0431 (assessment of oral abnormality). When dentists start using the product in the US after final FDA approval, they can use this code.
In March 2023, we were awarded FDA breakthrough device designation, a program by the FDA that designates the product as truly unique under the current standard. As we speak, there is legislation pending that all breakthrough products will get reimbursed. We hope for this legislation to come to fruition and also to have FDA approval in the US by summer 2024. When we receive approval, Henry Schein and Benco are ready to be distributors.
What will the future hold?
BB: The ability to improve is the beauty of a machine-learning algorithm. We hope to develop a future iteration that indicates how far along the cancer is on a scale.Interested clinicians go to vigilantbiosciences.com to learn more and be notified when available.
It’s a shame that basically about 73% of oral cancers get diagnosed late stage. And with those, there is a less than 50% 5-year survival rate.2 The signs should not be ignored until [the disease is] obvious. Vigilant Biosciences has given dentists the opportunity to save patients’ lives with early detection of oral cancer and make a big difference in healthcare.
Interested clinicians go to vigilantbiosciences.com to learn more and be notified when available.
