Lasers in Dentistry
Dental lasers provide an alternative to traditional treatments, are less invasive, and limit complications.
Shannon Pace Brinker, CDA, CDD

Since their introduction nearly 60 years ago, light amplification by stimulated emission of radiation (i.e., laser) devices have been widely used in the manufacturing, electronics, consumer, and medical industries.1
The first laser for dentistry-called the ruby laser-became available in 1964 and was used primarily for hard-tissue ablation, but it was eventually abandoned for dental hard-tissue preparation due to the thermal side effects of the laser light wavelengths, which increased temperatures in dental pulp, caused microcracks, and carbonization.2
In the 1980s, ultraviolet excimer lasers and infrared erbium lasers were developed to provide better-quality temperature control and shallower penetration depths. Smaller dental laser devices were introduced in the 1990s, enabling new ablation techniques that limited damage to surrounding tissues.2
Over the years, a variety of dental lasers have been introduced and are now used increasingly for a wide range of applications.3 In fact, applications for today’s dental lasers range from soft-tissue procedures, hard-tissue (i.e., tooth)
Angie Wallace, RDH

procedures, diagnostics, intraoral scanning for digital impressions, low-level laser therapy to relieve pain, provide wound healing, and tooth whitening, among others.4,5 In particular, specific dental laser devices are cleared by the FDA for certain applications. Broad clearance for soft-tissue surgery includes incision, excision, and coagulation of intraoral soft tissues. Other procedures range from treating herpetic lesions/aphthous ulcers to sulcular debridement, cementum-mediated new attachment, reducing bacterial level and inflammation, and pulpotomy. Cleared hard-tissue applications include composite curing, tooth whitening, carious lesion removal and tooth preparation, osseous surgery (e.g., osteotomy, bone shaping, osseous crown lengthening), and endodontic procedures (e.g., canal debridement, preparation, cleaning, apicoectomy).
Additionally, dental lasers have been instrumental in diagnostic applications for detecting carious lesions and calculus, as well as providing pain relief. For example, dental laser photobiomodulation (PBM) procedures are useful for temporarily increasing local blood circulation, and also temporarily relieving minor muscle and joint aches, pains, and stiffness.
However, each dental laser is cleared for use for only certain indications and according to specific techniques. These are determined by the dental laser technology’s specific wavelengths of laser light, which dictate the types of tissues (e.g., hard or soft) on which a particular device can be used.6 As a result, nearly every dental specialty-such as periodontics, endodontics, orthodontics, and oral and maxillofacial surgery-have dental lasers of different wavelengths available for use to facilitate appropriate procedures.7 A laser very good for use with one procedure may not be appropriate for another, since no single wavelength will treat all dental tissues.8
Therefore, it behooves dentists and their team members to understand their treatment objectives, what they want to accomplish, and different dental laser mechanisms of action before purchasing and subsequently using a dental laser in practice. This requires knowledge of a laser’s effects on tissue and how those affects are achieved.9,10
Categorizing dental lasers
Dental lasers are typically distinguished by their active medium and can contain gas, a crystal, or a solid-state semiconductor.10 The center of a laser device contains a cavity core, which comprises an active medium consisting of chemical elements, molecules, or compounds. When stimulated, it is the materials in the optical cavity core that produce specific laser light wavelengths.10 Different than ordinary light, lasers produce monochromatic light, with each wave identical in physical size and shape. The coherent, collimated, and monochromatic wave of light creates a uniquely efficient energy source.10
Dental laser wavelength
The wavelength of a laser’s light dictates its interactions, indications, and specificity for use in treatment procedures and for which types of tissues. Soft-tissue lasers’ wavelengths range within the visible light spectrum from argon at 488 nm, argon at 515 nm, to potassium titanyl phosphate at 535 nm.11 On the electromagnetic spectrum, near-infrared soft-tissue lasers’ wavelengths consist of 810 nm, 940 nm, 980 nm, and 1064 nm.11 Mid-infrared region lasers-such as those used for hard-tissue procedures-include wavelengths of 2,780 nm and 2,940 nm, and far–infrared region at 10,600 nm.4
The Waterlasse Express from BIOLASE is a portable all-tissue dental laser.

Four types of interactions (reflected, scattered, transmitted, and absorbed) and non-interactions occur between a laser and soft tissue. Lack of interaction occurs when the laser light beam reflects off of the surface, or when the light beam passes directly through the tissue, reflection and transmission occur, respectively.12 The most common laser interactions within soft tissue are absorption and scattering.13-15 Scattering occurs when the laser light disperses in a non-uniform way throughout the tissue.4 Absorption occurs when light radiation is absorbed by specific soft tissue elements, such as hemoglobin, melanin, water, and hydroxyapatite.4
Continue reading on the next page...
Once absorbed by the tissue, laser light energy produces a thermal reaction in that tissue. For example, ablation of soft tissue-which is composed of a high percentage of water-occurs at approximately 100ËC, the point of water vaporization.16,17 This is also the setting that hygienists use to reduce bacteria. In other words, as light energy enters into tissue, if it’s absorbed very readily on the surface, it will vaporize the tissue by raising the temperature until the water in the cells turns to steam, explode, and the tissue is removed.
Other thermal points can treat the tissue, such as at 50ËC and above, which inactivates non-sporulating bacteria,16 or at 60ËC, the point at which proteins begin to denature and coagulation occurs.17 To induce healing, the laser wavelength should not be readily absorbed, but rather penetrate deeper into the tissue to stimulate a biological response for healing.
Laser emission modes and delivery
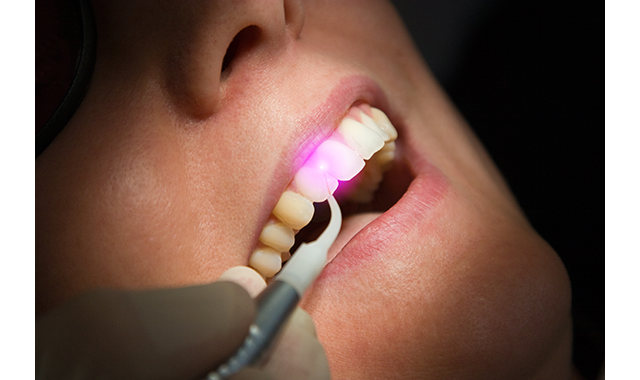
(Fig. 1) A dental hygienist utilizing Dentsply Sirona's SiroLaser Blue Diode for Laser Bacterial Reduction, or LBR.
(Fig. 1) A dental hygienist utilizing Dentsply Sirona's SiroLaser Blue Diode for Laser Bacterial Reduction, or LBR.In addition to wavelength, other attributes of dental lasers affect the applications for which they are indicated. These include whether or not a laser operates in continuous wave mode (i.e., the laser is on the entire time that the unit is powered on) or pulsed mode (i.e., the energy is released in bursts, sometimes known as gated or chopped mode); peak power; and ability to control the laser settings-both power and pulse. The average power in a continuous-wave laser ranges from 0.5 to 14 Watts.12 Free-running pulsed mode lasers produce a true pulse every few ten-thousandths of a second.10 These lasers exhibit high peak power, up to 1,000 Watts range, but deliver low average power through extremely short pulse durations every few hundred microseconds.12
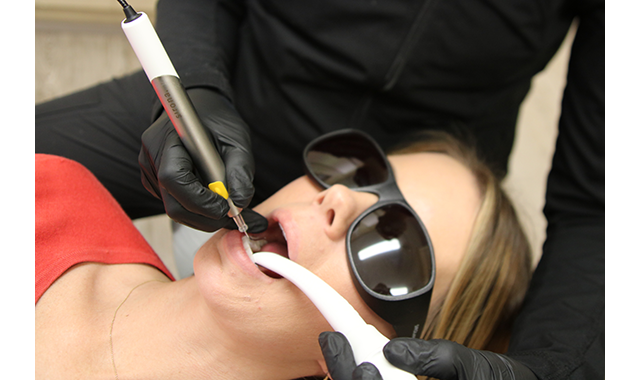
(Fig. 2) Utilizing Purvac HVE tip by Dentsply Sirona with a built-in dental mirror and HVE Hose. This enables a one-handed approach to evacuate the oral cavity of aerosols, splatter, fluid, and debris, while also providing visibility and illumination to the treatment area.
A dental laser can deliver its laser energy to tissue through one of several systems. Optic fibers, which are the most common delivery system, use small flexible glass fibers directly in contact with the tissue to deliver the energy.4 Semi-flexible, hollow waveguides and rigid sectional articulated arms are other delivery systems available.4,10 Some delivery systems also incorporate additional small quartz or sapphire tips that attach to the handpiece and contact the tissue.10
Types of dental lasers
Carbon Dioxide Lasers:
Used in dentistry for more than 25 years, carbon dioxide (CO2) lasers produce wavelengths of 10,600 nm and are ideal for incising and excising biopsies, frenectomies, and gingivectomies because their laser light readily interacts with free water molecules in the soft tissue and vaporizes the intercellular wall of pathogens.10 Although CO2 lasers incorporate continuous-wave emission mode, many also include gated-pulse interruption in either super-pulsed and micro-pulsed modes.11 CO2 lasers use waveguide and articulated arm delivery systems.4
The continuous-wave mode with gated pulse reduces the potential of thermal damage to the root surfaces, enabling CO2 laser use for periodontal therapy.11 The very short duration gated pulsing also provides precise control of the laser thermal action.18 Irradiation with a CO2 laser produces a positive effect on osteoblast proliferation and differentiation,31 and use of a CO2 laser in combination with augmentative techniques to treat peri-implant infrabony defects by decontaminating implant surfaces can be an effective way to address peri-implantitis.19 The Solea laser from Convergent Dental was the first CO2 dental laser to be cleared by the FDA for treatment on hard and soft tissue and operates at 9,300 nm.
Diode Lasers:
Diode lasers produce invisible near-infrared light at wavelengths of 810 nm, 940 nm, 980 nm, and 1,064 nm,4 and similar to CO2 lasers, diode lasers are continuous-wave emission mode that can also be manipulated into a gated pulse. Diode wavelengths specifically target the pigments in soft tissue, pathogens, and inflammatory and vascularized tissue.10
Effective for performing gingivectomies, biopsies, impression troughing, and frenectomies, diode lasers also produce bactericidal effects.5 Also, unlike CO2 lasers, diode lasers avoid creating surface defects on zirconia implants because they do not penetrate zirconia material.20 With a single application, diode lasers are also efficient in controlling inflammation around treated implants.21
Continue reading on the next page...
Erbium Lasers:
Erbium lasers (i.e., Er,Cr:YSGG and Er:YAG) produce wavelengths of 2,780 nm and 2,940 nm, respectively,5 and can effectively treat both hard and soft tissues;22 they are often referred to as all-tissue devices due to their short temporal emission and laser light absorption by hard and soft tissue. Used for periodontal procedures, gingival contouring, biopsies, frenectomies, and pre-prosthetic procedures,5 these lasers provide higher powers and more efficient delivery systems for faster cutting speeds and improved hemostasis.11 With free-running pulsed emission mode, erbium lasers deliver square pulses rather than bell-curved analog pulses, resulting in quick ablation and reduced pain.11 Erbium lasers also create photo acoustic shockwaves to increase efficient debridement of endodontic canals.5
Used as a single treatment, or for complementing surgical procedures,23 erbium lasers ablate soft tissue, hard tissue, and biofilms while avoiding thermal damage to the adjacent tissue.22 Use of Er:YAG lasers can eliminate the need for local anesthesia, minimize post-operative pain, and decrease recovery time.24 Additionally, erbium laser irradiation may increase osteoblast proliferation and differentiation.25
Further, Er:YAG lasers can be used to successfully debride/decontaminate implant surfaces,23 regenerate osseous tissues without complications but with high patient and clinician satisfaction,26 and treat peri-implantitis, resulting in improvements in plaque index, bleeding on probing, probing depth, gingival recession, inflammation, and clinical attachment level.27
Nd:YAG Lasers:
Developed for use in periodontal treatments, Nd:YAG lasers produce wavelengths in the near-infrared range of 1,064 nm and target the pigments in the soft tissue, primarily hemoglobin and melanin.5 With free-running pulsed emission mode, Nd:YAG lasers have various pulse increments for transitioning from cutting to hemostasis.28 Ideal for gingivectomy, frenectomy, impression troughing, biopsy, debridement, and disinfection, Nd:YAG lasers may also be used for bacterial ablation and reducing implant contamination, without damaging the surface properties.29
Additionally, surgical and non-surgical Nd:YAG laser treatments may even be performed without local anesthesia, enabling full-mouth treatment in one appointment and with minimal bleeding, reduced post-operative pain, no swelling, and no sutures.30 Due to these benefits, patient acceptance of laser therapy is greater than for conventional treatments because Nd:YAG laser approaches require about 50 percent less time.
Soft-tissue laser procedures
Within surgical procedures, good hemostasis and reduced need for sutures is achieved when soft-tissue lasers are used. Other benefits include selective and exact interaction with tissues-which results in less trauma, ability to lower the bacterial load in the surgical field, reduced inflammation, and stimulation of new fibroblasts and osteoblasts for improved healing.10
Soft-tissue lasers have also been instrumental for managing gingival tissues for esthetic treatment, non-surgical and surgical periodontal pocket therapy, osseous surgery, and implant therapy.31,32 What’s more, compared to a scalpel, lasers produce excellent coagulation, reduce pathogens, temporarily seal nerve endings and lymphatic vessels, and potentially eliminate the need for sutures, all with less scar formation.
Additionally, soft-tissue dental lasers are beneficial in helping to manage periodontal disease.31 Various soft-tissue laser wavelengths for treating chronic periodontitis-in addition to conventional scaling and root planning-have been shown to effectively reduce probing depth and subgingival bacterial populations.5
Continue reading on the next page...
Hard-tissue laser procedures
Erbium lasers can prepare enamel, dentin, caries, cementum, and bone. In addition, erbium and other hard-tissue lasers can be used for removing bone in crown-lengthening procedures for esthetic enhancements.10 They can also cut soft tissue.33 When used for hard-tissue applications, erbium lasers diminish or eradicate vibrations, the sound of dental drills, microfractures, and alleviate a great deal of stress that patients associate with high-speed handpieces.33 Restoratively speaking, and compared to a drill, hard-tissue lasers produce no smear layer on dentin or bone, no microfracturing of enamel rods, and simultaneously disinfect hard-tissue surfaces.
(Fig. 3) Lynn Atkinson utilizing the BIOLASE Epic Laser to perform laser-assisted periodontal therapy.
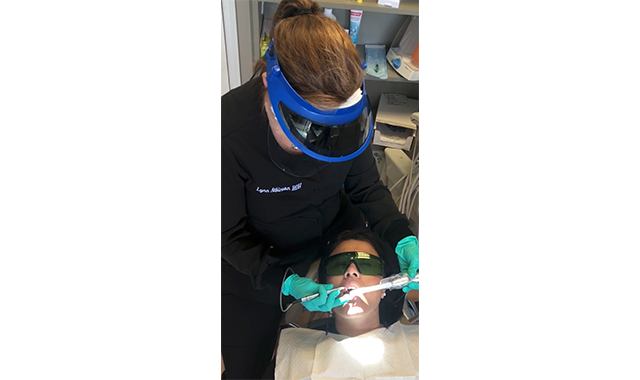
In fact, hard-tissue lasers have been used in conjunction with endodontics since the early 1970s. Laser applications within endodontics include pulp diagnosis, treating dental hypersensitivity, pulp capping and pulpotomy, root canal sterilization, root canal shaping, and obturation and apicectomy.34
Low-level laser procedures
Low-level laser therapy (LLLT) or PBM, has been a treatment modality for the past 30 years. Low-level laser therapy or cold laser therapy is used for wound healing and muscle repair. LLLT also has FDA clearance for treating and managing oral mucositis.
LLLT uses light energy from adenosine triphosphate (ATP) to stimulate the body’s biological responses. This increases cellular energy and alterations in the cell membrane, resulting in pain relief, wound healing, muscle relaxation, immune system modulation, and nerve regeneration.35
Although low-level laser therapy has been used with soft tissues, it is also being used increasingly for hard-tissue applications, such as treating dentin hypersensitivity and pain attributed to the periodontal ligament.36 In fact, research has demonstrated that laser therapy is advantageous compared to topical medicaments when treating dentin hypersensitivity.37
Laser diagnostic procedures
Since their introduction, diagnostic lasers have increasingly become a part of routine clinical examinations. Fiber-optic transillumination is visible to the naked eye, showing changes in color, shadowing, and craze lines within enamel and dentin.38 Digital imaging fiber-optic transillumination incorporates a transilluminating camera to identify demineralization and capture images of the illuminated tooth using visible light. The examination can be performed in real time, with the camera moved around a patient’s mouth. This technique also can determine changes in density, even in interproximal areas.39
Camera’s such as the Digital Doc’s LUM extends the detection capabilities of America’s leading camera IRIS with well-proven sub-enamel illumination diagnostic methods. This compact, easy-to-use tool provides instant documentation as well as detection of all findings. The LUM’s specialized LED technology applies a high-intensity light source to the tooth with unique positioning so the light is traveling perpendicular to the plane of the tooth.
In a tooth without impurities the light will travel uninterrupted from the buccal surface to the lingual, which can be observed on the occlusal table of the tooth by its uniform illumination. When a tooth has impurities such as fractures or leaking amalgam, the light is dispersed showing up clearly in near X-ray like images.
Laser-induced fluorescence, however, is often considered the benchmark for cavity detection. These devices direct laser light at hard dental tissues in order to identify if there will be a shift in the light wavelength being reflected back from the tooth, depending on the nature and density of the tissue. Typically, a 655-nm cavity detection laser uses fluorescence to quantify the presence of decay, help identify pit-and-fissure lesions, and provide quantitative data.
More recently introduced caries detection lasers rely on light emitting diode (LED) fluorescence, filters and image-capturing technology within the unit to differentiate between decay and demineralized tooth structure. Differently colored areas indicate the progression of carious lesions or the effectiveness of remineralization therapy.39
Education and use requirements
As with all technologies, it is imperative that dental professionals undergo thorough and comprehensive training in order to understanding the functionalities, limitations, and applications of dental laser technologies. It’s also essential that the entire staff is appropriately trained on the safe and effective use of dental lasers, as well as an understanding of safety standards and government regulations.
Digital Doc's LUM intraoral camera uses LED to apply a high-intensity light to the tooth.

Digital Doc's LUM intraoral camera uses LED to apply a high-intensity light to the tooth.Among the important considerations for dental practices to follow when incorporating lasers is the requirement of having a designated Laser Safety Officer (LSO). The LSO is responsible for establishing the office’s laser procedural and administrative controls and protocols to ensure the laser is used in a safe and effective manner. Some of the primary duties of the LSO include defining the hazard zone when a laser is in use and ensuring that all of the practice’s staff members have been adequately educated and trained on laser use and safety to the appropriate level for their duties. The LSO’s other responsibilities include-but are not limited to-regular inspection of the laser system(s) in use; ensuring laser protective eyewear is appropriate and in good repair; and ensuring standards for laser safety and infection control protocols are followed.
Continue reading on the next page...
Although each state’s dental practice act regarding lasers varies in terms of certification, training, and who can use a laser for which procedures, licensed dental professionals must be properly trained and use a laser within their scope of practice, as well as in a manner that is safe, effective, and consistent with the clinician’s education, training, and experience.
Hands-on training should focus specifically on the device the clinician is using and the procedures being performed. Regulations on whether a dental hygienist may or may not use a laser, and the type of training required, also varies from state to state.
Conclusion
From soft-tissue lasers to those designed for hard-tissue applications, and from lasers used for dental diagnostics to those approved for therapeutic indications, dental lasers today can serve many beneficial roles when used by properly trained professionals.
An alternative to conventional treatment modalities, laser treatments reduce the number of necessary appointments and stress, increase visibility, improve patient comfort, and reduce complications. As with any less-invasive treatment, laser therapy eliminates many of the complications associated with surgical treatment, providing regeneration effects.
Training, certification, and information on specific state regulations can be obtained from the Academy of Laser Dentistry (ALD) at laserdentistry.org. The ALD’s annual meeting this year will be held April 2-4 in San Diego.
References
1. Myers TD, McDaniel JD. The pulsed Nd: YAG dental laser: Review of clinical applications. J Calif Dent Assoc. 1991;19(11):25-30.
2. De Moor RJ, Delmè KL. Laser-assisted cavity preparation and adhesion to erbium-lased tooth structure: Part 1. Laser-assisted cavity preparation. J Adhes Dent. 2009;11(6):427-438.
3. Najeeb S, Khurshid Z, Zafar MS, Ajlal S. Applications of Light Amplification by Stimulated Emission of Radiation (Lasers) for Restorative Dentistry. Med Princ Pract. 2016;25(3):201-11.
4. Pang P, Andreana S, Aoki A, et al. Laser energy in oral soft tissue applications. J Laser Dent. 2010;18(3):123-31.
5. DiMatteo AM, Rafferty T. Dental lasers. Inside Dent. 2012;8(11):112-118.
6. Sulieman M. An overview of the use of lasers in general dental practice: 2. Laser wavelengths, soft and hard tissue clinical applications. Dent Update. 2005;32(5):286-296.
7. Green J, Weiss A, Stern A. Lasers and radiofrequency devices in dentistry. Dent Clin North Am. 2011;55(3):585-597.
8. Fasbinder DJ. Digital dentistry: innovation for restorative treatment. Compend Contin Educ Dent. 2010;31(8 Special Issue 1):2-12.
9. Magid KS, Strauss RA. Laser use for esthetic soft tissue modification. Dent Clin North Am. 2007;51(2):525-545.
10. Coluzzi DJ. Fundamentals of lasers in dentistry: basic science, tissue interaction, and instrumentation. J Laser Dent. 2008;16(special issue):4-10.
11. Swick M. Dental lasers. Inside Dent. 2010;6(7):122.
12. Kydd WL, Daly CH, Wheeler JD 3rd. The thickness measurement of masticatory mucosa in vivo. Int Dent J. 1971;21(4):430-41.
13. Gillis TM, Strong MS. Surgical lasers and soft tissue interactions. Otolaryngol Clin North Am. 1983;16(4):775-84.
14. Moshonov J, Stabholz A, Leopold Y, et al. [Lasers in dentistry. Part B–Interaction with biological tissues and the effect on the soft tissues of the oral cavity, the hard tissues of the tooth and the dental pulp.] [Article in Hebrew.] Refuat Hapeh Vehashinayim. 2001;18(3-4):21-8, 107-8.
15. Ball KA. Lasers: The perioperative challenge. 3rd ed. Denver, Colorado: AORN, 2004:14-18.
16. Russell AD. Lethal effects of heat on bacterial physiology and structure. Sci Prog. 2003;86(Pt 1-2):115-37.
17. Knappe V, Frank F, Rohde E. Principles of lasers and biophotonic effects. Photomed Laser Surg. 2004;22(5):411-7.
18. Lasers. Inside Dent. 2011;7(7):90.
19. Romanos GE, Nentwig GH. Regenerative therapy of deep peri-implant infrabony defects after CO2 laser implant surface decontamination. Int J Periodontics Restorative Dent. 2008;28(3):245-55.
20. Stübinger S, Homann F, Etter C, et al. Effect of Er:YAG, CO(2) and diode laser irradiation on surface properties of zirconia endosseous dental implants. Lasers Surg Med. 2008;40(3):223-8.
21. Kotsakis GA, Konstantinidis I, Karoussis IK, et al. A systematic review and meta-analysis of the effect of various laser wavelengths in the treatment of peri-implantitis. J Periodontol. 2014 Jan 20. [Epub ahead of print].
22. Kulakov AA, Khamraev TK, Kasparov AS, et al. [Use of erbium laser for treatment of dental implant complications]. Stomatologiia (Mosk). 2012;91(6):55-8.
23. Badran Z, Bories C, Struillou X, et al. Er:YAG laser in the clinical management of severe peri-implantitis: a case report. J Oral Implantol. 2011;37 Spec No:212-7.
24. Arnabat-Domínguez J, España-Tost AJ, Berini-Aytés L, et al. Erbium:YAG laser application in the second phase of implant surgery: a pilot study in 20 patients. Int J Oral Maxillofac Implants. 2003;18(1):104-12.
25. Lee JH, Heo SJ, Koak JY, et al. Cellular responses on anodized titanium discs after laser irradiation. Lasers Surg Med. 2008;40(10):738-42.
26. Azzeh MM. Er,Cr:YSGG laser-assisted surgical treatment of peri-implantitis with 1-year reentry and 18-month follow-up. J Periodontol. 2008;79(10):2000-5.
27. Schwarz F, Bieling K, Nuesry E, et al. Clinical and histological healing pattern of peri-implantitis lesions following non-surgical treatment with an Er:YAG laser. Lasers Surg Med. 2006;38(7):663-71.
28. Nicholson D, Blodgett K, Braga C, et al. Pulsed Nd:YAG laser treatment for failing dental implants due to peri-implantitis. Proc. SPIE 8929. Lasers in Dentistry XX, 89290H. (February 18, 2014); doi. 10.1117/12.2041037.
29. GonÒ«alves F, Zanetti AL, Zanetti RV, et al. Effectiveness of 980-mm diode and 1064-nm extra-long-pulse neodymium-doped yttrium aluminum garnet lasers in implant disinfection. Photomed Laser Surg. 2010;28(2):273-80.
30. Lioubavina-Hack N. [Lasers in dentistry. 5. The use of lasers in periodontology] [Article in Dutch]. Ned Tijdschr Tandheelkd. 2002;109(8):286-92.
31. Aoki A., Mizutani K., Takasaki AA, et al. Current status of clinical laser applications in periodontal therapy. Gen Dent. 2008;56(7):674-687.
32. Fasbinder DJ. Dental laser technology. Compend Contin Educ Dent. 2008;29(8):452-459.
33. van As G. Erbium lasers in dentistry. Dent Clin North Am. 2004;48(4):1017-1059.
34. Kimura Y, Wilder-Smith P, Matsumoto K. Lasers in endodontics: a review. International Endodontic Journal. 2000;33:173-185.
35. Ross G, Ross A. Low level lasers in dentistry. Gen Dent. 2008;56(7):629-634.
36. Walsh LJ. The current status of low level laser therapy in dentistry. Part 1. Soft tissue applications. Aust Dent J. 1997;42(4):247-254.
37. Cunha-Cruz J. Laser therapy for dentine hypersensitivity. Evid Based Dent. 2011;12(3):74-75.
38. Strassler HE, Sensi LG. Technology-enhanced caries detection and diagnosis. Compendium Contin Educ Dent. 2008;29(8):464-470.
39. Kutsch K. Caries detection technologies. Inside Dentistry. 2012;8(5):74-78.

ACTIVA BioACTIVE Bulk Flow Marks Pulpdent’s First Major Product Release in 4 Years
December 12th 2024Next-generation bulk-fill dental restorative raises the standard of care for bulk-fill procedures by providing natural remineralization support, while also overcoming current bulk-fill limitations.