Catapult Review: A Novel Implant Cement Universally Impresses
Ceramir Bioceramic Implant Cement earns unanimous praise from Catapult Evaluation panel with its biocompatibility, quick clean up, and easy handling.

With an ever-increasing number of implants being placed and restored in dentistry, the need for a reliable luting material cannot be denied.
Implant restorations present the clinician with very different concerns than those encountered with tooth-borne restorations. The lack of traditional gingival architecture surrounding dental implants creates an increased risk for cement retention in the peri-implant sulcus which can lead to an inflammatory response and ultimately peri-implantitis.
At the same time, retrievability is seen as a favorable trait in an implant luting material whereas most clinicians do not consider ease of crown removal for tooth-borne restorations.
In the spring of 2019, Doxa Dental requested that Catapult Education evaluate an innovative product that addresses all of the above concerns-Ceramir® Bioceramic Implant Cement.
About the product
Ceramir Bioceramic Implant Cement is a permanent, radiopaque bioceramic cement with excellent handling properties. Ceramir Implant Cement is 100 percent resin-free and self-setting. It was purposefully engineered to provide good flowability and easy seating. Bioceramic technology is the key element behind the active ion exchange seen with Ceramir Implant Cement, and this also allows for a tight seal for ceramics and metals alike. By combining these qualities with an unprecedented ease of clean up, Doxa is confident in the clinical applicability of this product.
The evaluation
Twenty of Catapult Education’s clinicians participated in the evaluation over the third and fourth quarters of 2019, after which they were surveyed to gain user perspective on the implant cement. Doxa Dental did not compensate the evaluators for their participation, nor was there any form of compensation to the clinicians.
The results
Most of the evaluators were already familiar with the chemistry and handling characteristics of Ceramir Cement with an impressive 60 percent of the evaluators already using this material for implant crown cementation. Of the current users, half reported that Ceramir was their exclusive implant crown luting agent of choice, while the other half reported having an alternative for varying indications such as short abutments or esthetic protocols.
Interestingly, only one other single brand had multiple users amongst our evaluators, that being BISCO’s Theracem with three users. Of the eight evaluators that were not currently using Ceramir, seven different products were being utilized in differing material categories as follows;
Resin Modified Glass Ionomer – 2; Implant Cements – 2; Theracal – 2; ZO-P – 1; ZO-E – 1
Continue reading on the next page...
(Fig. 1) Zirconia abutments torqued to place.
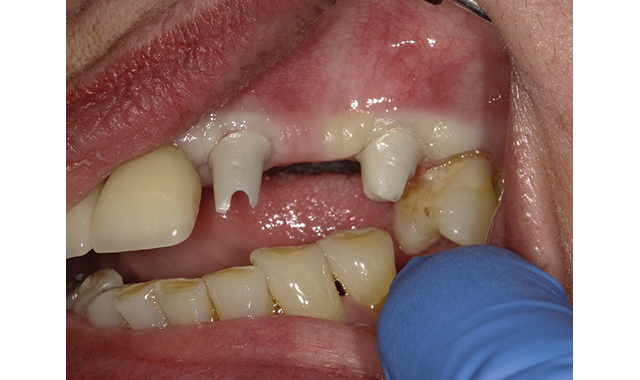
Ease of clean-up: Excess cement retention around implant-retained crowns is a primary concern for the restorative dentist. This was confirmed by asking our evaluators to rank required implant cement characteristics in order of importance. Overwhelmingly, nearly 90 percent of evaluators ranked ease of cement removal and clean-up as the most important attribute with marginal integrity as a distant second. The Catapult evaluators all rated this product among the best-in-class regarding ease of excess removal.
Biocompatibility: When asked about the most important characteristics, it must be noted that biocompatibility was not one of the survey choices, though it is one of Ceramir’s most desirable characteristics. This was confirmed in our survey as 66 percent of respondents reported biocompatibility as Ceramir’s best attribute in a separate survey question.
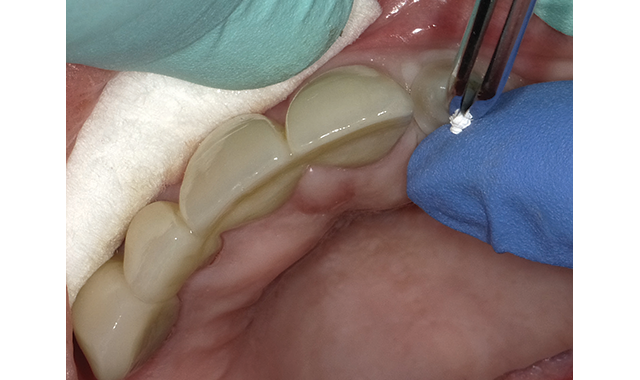
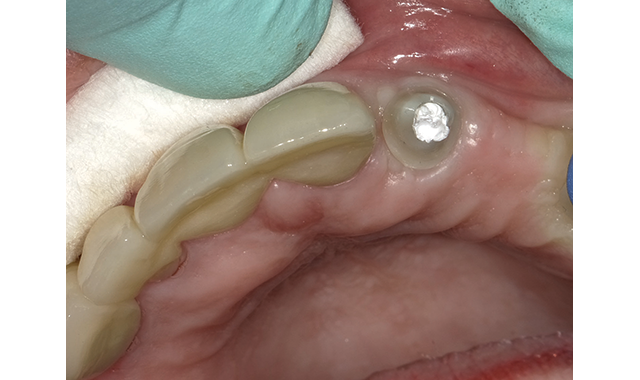
(Figs. 2-3) Teflon Tape place to protect the abutment screw.
Technique: When asked about their preferred implant cementation technique, 18 of the 20 evaluators stressed the need to minimize excess cement prior to final cementation over the abutment. To this point, almost half of the clinicians surveyed took the time to create an analog abutment, first seating the restoration over the analog to prevent excess cement on the intaglio surface. The remaining clinicians used either a brush or instrument to control excess. The takeaway here is that while biocompatibility is incredibly beneficial to the peri-implant sulcus, removal of excess cement still remains a priority.
Continue reading on the next page...
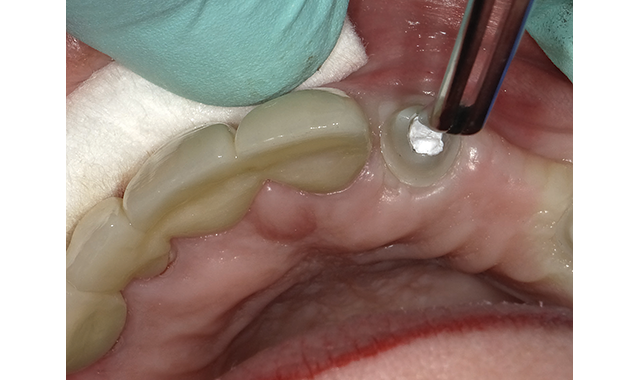
(Fig. 4) Teflon condensed with amalgam condenser below level of access opening.
Handling characteristics: In the categories of thixotropic properties, film thickness, working time and setting time, more than 80 percent of those surveyed rated Ceramir in the two most favorable columns with no responses at the opposite end of the spectrum. The only area that showed potential for improvement was regarding the convenience of use for Ceramir cement. While 75 percent of the evaluators felt that the material was either convenient or very slightly inconvenient, one-fourth saw some opportunity for improvement. Despite this, the value of this material to our clinicians was made very apparent with every single respondent reporting that Ceramir Bioactive Implant Cement is a product they would incorporate into their clinical formulary. This should not be taken lightly as getting 100 percent agreement from a group of dentists on any matter is quite impressive.
(Fig. 5) A layer of Ceramir Cement is placed and thinned with a brush. The crown is then seated.
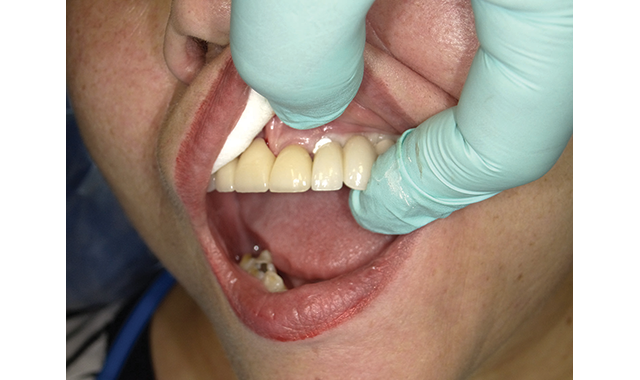
Suggested Improvements: Of the Catapult Evaluators, 20 percent responded that Ceramir ranked fair or less than fair regarding convenience, mainly regarding the dispensing system. The only other comment regarding dispensing suggested that the amount of force required to activate the capsule prior to trituration may be excessive.
(Fig. 6) Crown in place for 2 minutes, allowing for simple clean up.
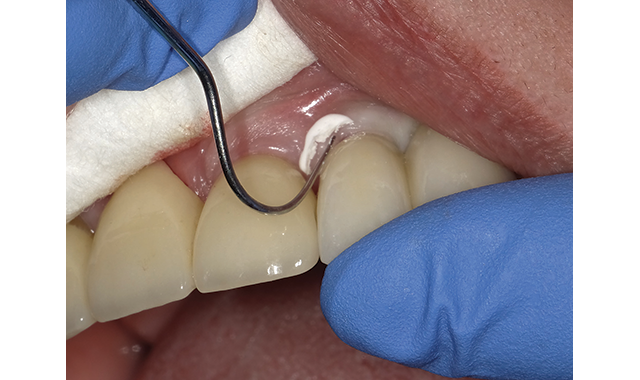
Plan to purchase & use in practice: Every single evaluator from this panel of 20 plan on incorporating Ceramir into their practices. Only one felt that changes to the delivery system would be necessary to keep them from using a similar product if and when it became available.
Continue reading on the next page...
Final thoughts
Rarely have the responses from this group of clinicians been so unanimously favorable for any product. The overwhelmingly positive response to Ceramir Bioactive Implant Cement from our evaluators has earned Doxa Dental and this innovative product the Catapult Vote of Confidence.
While it must be noted that the high percentage of current users in our evaluator group may not be representative of the dental population at-large, it is absolutely a testament to the unique qualities of this product that every Catapult clinician who has tried Ceramir continues to use it.
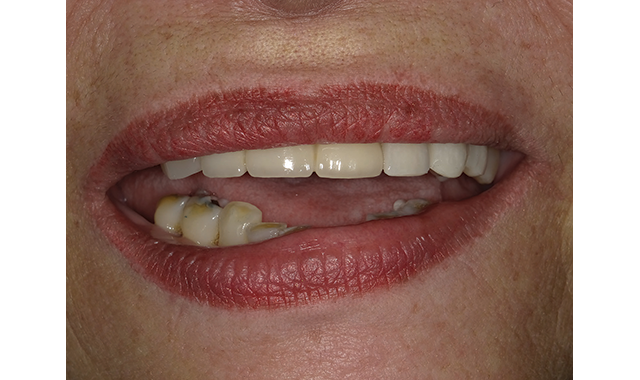
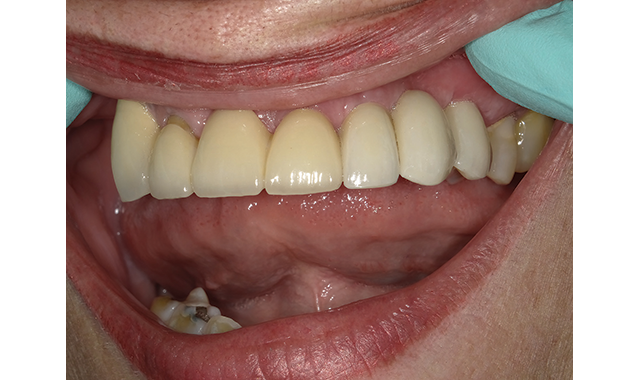
(Figs. 7-8) Implant crown in place following an easy final cleanup.
This will undoubtedly translate to the at-large dental community. This can be further supported by the vast number of different products used by our evaluators who have the opportunity to communicate and collaborate. Only one other brand had multiple users showing that there is no agreed-upon standard for implant cementation.
What is common knowledge, is that meticulous cement removal is a must around implants. This evaluation clearly supports that Ceramir is a leader in the ease of clean-up category.
What is also known is that, despite our best efforts and intentions, small amounts of cement may be left by even the best of clinicians. To reduce the risks posed by this common occurrence, Ceramir boasts unprecedented biocompatibility.
Knowing how common excess cement retention is, why would any clinician not use a product like Ceramir that makes it easier to clean up while being better tolerated by the surrounding tissues? Given the current material choices, Ceramir could become the gold standard for implant crown cementation, carving out a true niche in implantology that no other has yet claimed.
About Catapult

Catapult is an organization that consists of more than 50 clinicians spread throughout Canada and the United States. As a company, manufacturers pay a fee for their product to be evaluated and what we deliver are truthful, independent answers from surveys that we develop with them. We have had many products that have either had to be altered before hitting the market or simply never arrived because of our openly honest evaluations. In this way, Catapult assists the manufacturer to avoid potentially releasing a faulty product or simply a product that needs refinement. Lastly, our clients are omnipresent in the industry, small to large, no favoritism, simply reviewing the latest products in our practices.

ACTIVA BioACTIVE Bulk Flow Marks Pulpdent’s First Major Product Release in 4 Years
December 12th 2024Next-generation bulk-fill dental restorative raises the standard of care for bulk-fill procedures by providing natural remineralization support, while also overcoming current bulk-fill limitations.