X-Nav receives FDA clearance for X-Mark technology
X-Mark is a new virtual patient registration technology that advances navigated dental implant surgery.
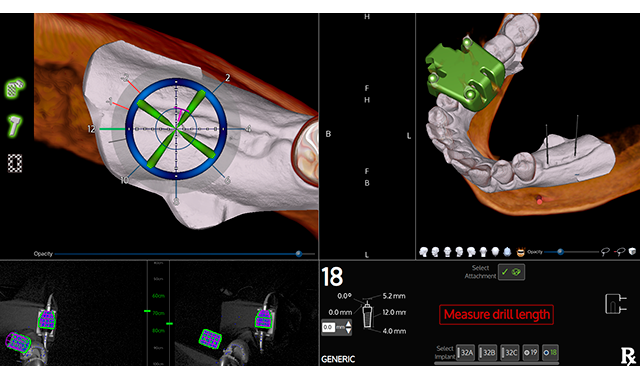
X-Nav Technologies, LLC has received 510(k) clearance from the Food and Drug Administration (FDA) for X-Mark™, a new virtual patient registration technology that advances navigated dental implant surgery, the company announced in a press release.
X-Mark enhances the company’s X-Guide Dynamic 3D Navigation system to give dentists the ability to deliver more accurate dental implant procedures to more patients seeking new teeth.
X-Mark features technology to facilitate virtual-based registration of the patient’s anatomy to a digital treatment plan. Developed to be fast and easy, the dentist will prepare for surgery by marking three virtual anatomy points on the patient’s 3D scan and then mark the same three live points on the patient at the time of the surgery. The X-Guide system will use the X-Mark technology to quickly match the points together to enable live navigated surgery.
The system is designed to work in a variety of applications, from single tooth replacements to full mouth edentulous reconstruction. The X-Guide system is said to fit seamlessly into any practice and work with all cone beam 3D scanners, including small field of view.
“Over the past few years, the global dental industry has rapidly embraced the X-Guide dynamic 3D navigation technology because of its ability to deliver more accurate dental implant outcomes,” said Edward Marandola, president of X-Nav Technologies. “X-Mark virtual registration continues to advance and streamline the way surgeons place dental implants- while improving accuracy and facilitating better immediate restorative results. I am excited to launch X-Mark to expand the capabilities of the X-Guide system so our customers can more easily offer navigation surgery to more patients.”
X-Guide will also allow the surgeon to visualize precise 3D movements of the handpiece during osteotomy and implant delivery for more exact placement. This is said to deliver better functional and esthetic results.
X-Mark for X-Guide is reportedly the industry’s first virtual patient registration process for dynamic dental navigation to receive 510(k) clearance from the FDA. X-Mark has also received CE Mark approval from the European Community, and Health Canada Medical Device License approval.
For more information, visit x-navtech.com.
Oral Health Pavilion at HLTH 2024 Highlighted Links Between Dental and General Health
November 4th 2024At HLTH 2024, CareQuest, Colgate-Palmolive, Henry Schein, and PDS Health launched an Oral Health Pavilion to showcase how integrating oral and general health can improve patient outcomes and reduce costs.
Episode 31: Dentsply Sirona Implant Announcements
September 30th 2021DPR’s Editorial Director Noah Levine sat down with Gene Dorff, Dentsply Sirona’s group vice president of implants and Dr. Dan Butterman to review several big announcements the company made in the arena of implants during Dentsply Sirona World 2021 in Las Vegas.
Oral Health Pavilion at HLTH 2024 Highlighted Links Between Dental and General Health
November 4th 2024At HLTH 2024, CareQuest, Colgate-Palmolive, Henry Schein, and PDS Health launched an Oral Health Pavilion to showcase how integrating oral and general health can improve patient outcomes and reduce costs.
Episode 31: Dentsply Sirona Implant Announcements
September 30th 2021DPR’s Editorial Director Noah Levine sat down with Gene Dorff, Dentsply Sirona’s group vice president of implants and Dr. Dan Butterman to review several big announcements the company made in the arena of implants during Dentsply Sirona World 2021 in Las Vegas.
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.