Vivos Therapeutics’ Sleep Apnea mmRNA Oral Appliance Receives FDA Market Clearance
The sleep apnea appliance has received FDA market clearance which will allow it to be more widely used and covered by insurance.
Vivos Therapeutics
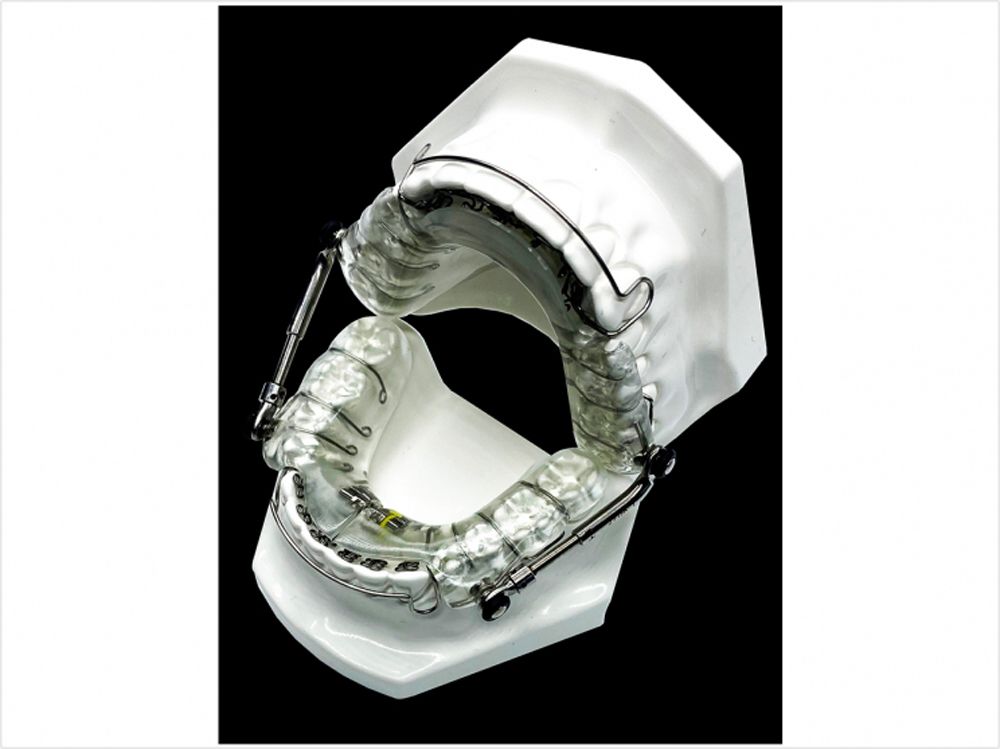
Medical technology company Vivos Therapeutics has received 510(k) market clearance for its modified mandibular Repositioning Nighttime Appliance (mmRNA) in treatment of mild to moderate sleep-disordered breathing in adults.
Vivos Therapeutics specializes in research and development of treatment modalities for patients who deal with sleep apnea and other sleep-disordered breathing. The mmRNA is the latest modality granted clearance by the U.S. Food and Drug Administration (FDA). The FDA clearance will allow the device to be reimbursed by insurance coverage which includes Medicare. Kirk Huntsman, Vivos Chairman and CEO, said in a press release that this clearance will greatly improve the lives of those who suffer from sleep-disordered breathing.
“The FDA’s market clearance of Vivos’ newest device, the mmRNA appliance, represents a significant milestone in our ongoing efforts to provide the best possible treatment for people who continue to suffer needlessly from obstructive sleep apnea (OSA), a debilitating condition that causes or contributes to a wide range of chronic health issues,” Huntsman said in the press release.
With OSA being a problem for 1 billion people globally, Vivos’s aim is to serve those who suffer from this chronic illness through a range of oral appliances. According to the press release, patients treated with the Vivos System rarely require lifetime intervention after treatment.
In addition to this news, Vivos’s previously submitted DNA appliance has been denied by the FDA. Already registered with the FDA as a Class I device, this appliance will be resubmitted with an appeal of the FDA’s decision. The appliance is still usable, and Vivos is optimistic about future prospects and outcomes.
ACTIVA BioACTIVE Bulk Flow Marks Pulpdent’s First Major Product Release in 4 Years
December 12th 2024Next-generation bulk-fill dental restorative raises the standard of care for bulk-fill procedures by providing natural remineralization support, while also overcoming current bulk-fill limitations.