ProSomnus Announces EVO Sleep and Snore Device Qualifies for Medicare Reimbursement
ProSomnus’s EVO Sleep and Snore Device will now qualify for Medicare reimbursement, allowing beneficiaries to use this device to manage obstructive sleep apnea.
ProSomnus Announces EVO Sleep and Snore Device Qualifies for Medicare Reimbursement
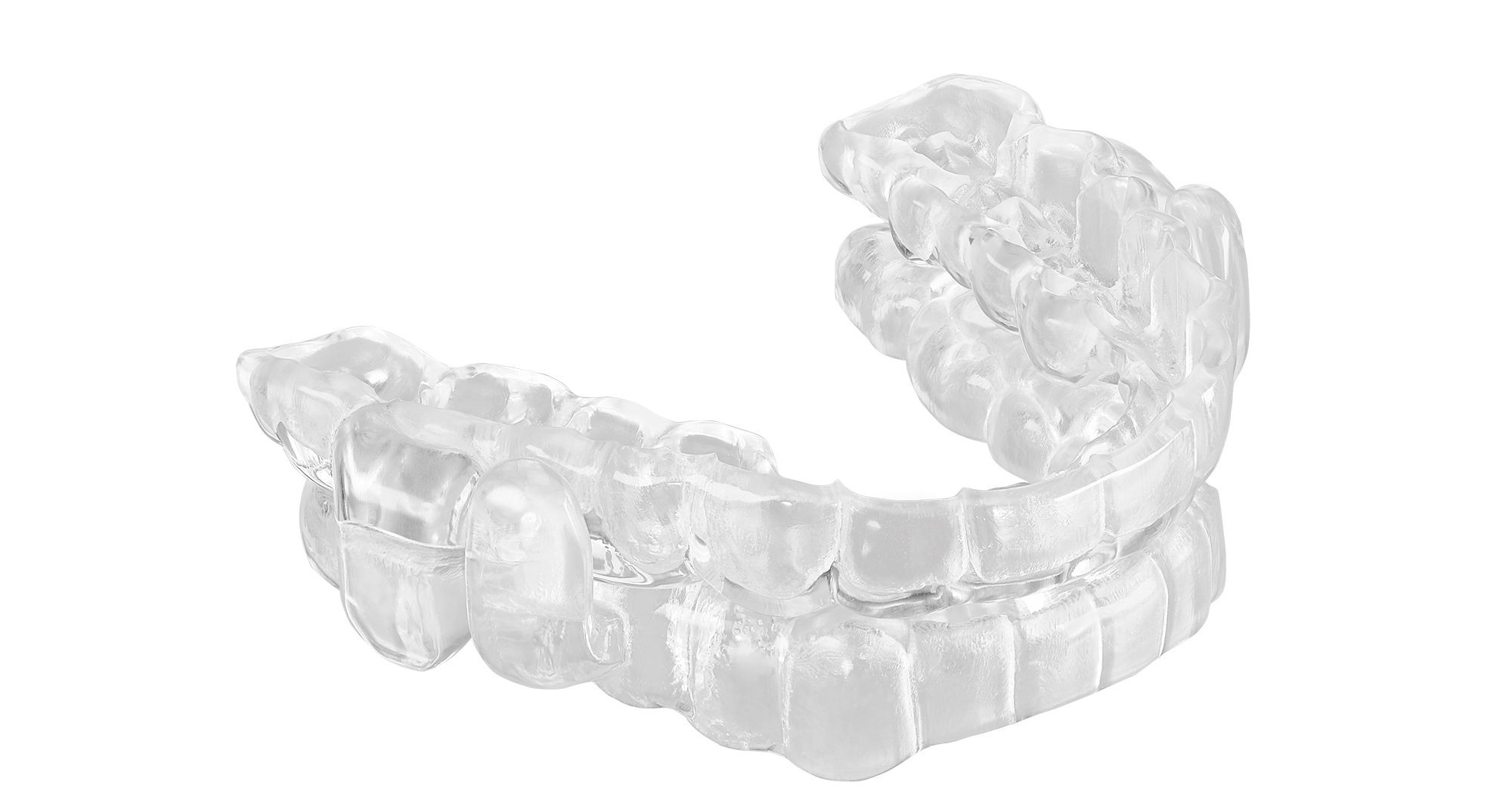
Oral appliance therapy company ProSomnus has announced that its ProSomnus EVO® Sleep and Snore Device now qualifies for Medicare reimbursement. The EVO has been reviewed and verified by The Pricing, Data Analysis, and Coding Contractor for Procedure Coding System code E0486. In November of 2022, ProSomnus received 510(k) clearance from the Food and Drug Administration for the EVO. Healthcare beneficiaries will benefit greatly from this reimbursement, according to Co-Founder and CEO of ProSomnus Len Liptak.
“With Medicare coding verification, the ProSomnus EVO offers healthcare providers and their Medicare beneficiary patients a comfortable, effective, and reimbursable treatment option for obstructive sleep apnea (OSA) that addresses many of the limitations of dental product and CPAP therapies,” Liptak said in a press release from the company. “Achieving Medicare coding verification for the ProSomnus EVO enhances our momentum as we expand availability of precision OAT to even more patients, thereby increasing adoption and creating better patient outcomes when treating OSA, a disease impacting 74 million Americans today.”
The ProSomnus EVO is designed to reposition and stabilize the patient’s jaw overnight while they sleep. This increases pharyngeal space and reduces risk of upper airway collapse, according to ProSomnus. The device is said to be 32% smaller than other devices for patient/beneficiary comfort, and is specifically designed to meet Centers for Medicare Services coding guidelines. After a study done by ProSomnus, 91% of patients strongly preferred the ProSomnus EVO to their prior therapy, 94% of patients reported that EVO was more comfortable, 100% of providers said they would prescribe the device again, and 100% of providers said they would recommend it to their colleagues.
“The ProSomnus EVO addresses some of providers’ and patients’ biggest concerns regarding legacy dental product therapies, including flexibility, durability, stain resistance, and maintaining form,” Co-Founder and Chief Technology Officer of ProSomnus, Sung Kim said in the press release. “Designing a user-friendly yet high-performance device with high quality materials is key as we continue to raise awareness of precision OAT as a patient-preferred OSA treatment, and in turn addressing the global health emergency.”
ACTIVA BioACTIVE Bulk Flow Marks Pulpdent’s First Major Product Release in 4 Years
December 12th 2024Next-generation bulk-fill dental restorative raises the standard of care for bulk-fill procedures by providing natural remineralization support, while also overcoming current bulk-fill limitations.