Adravision Perio from Adravision
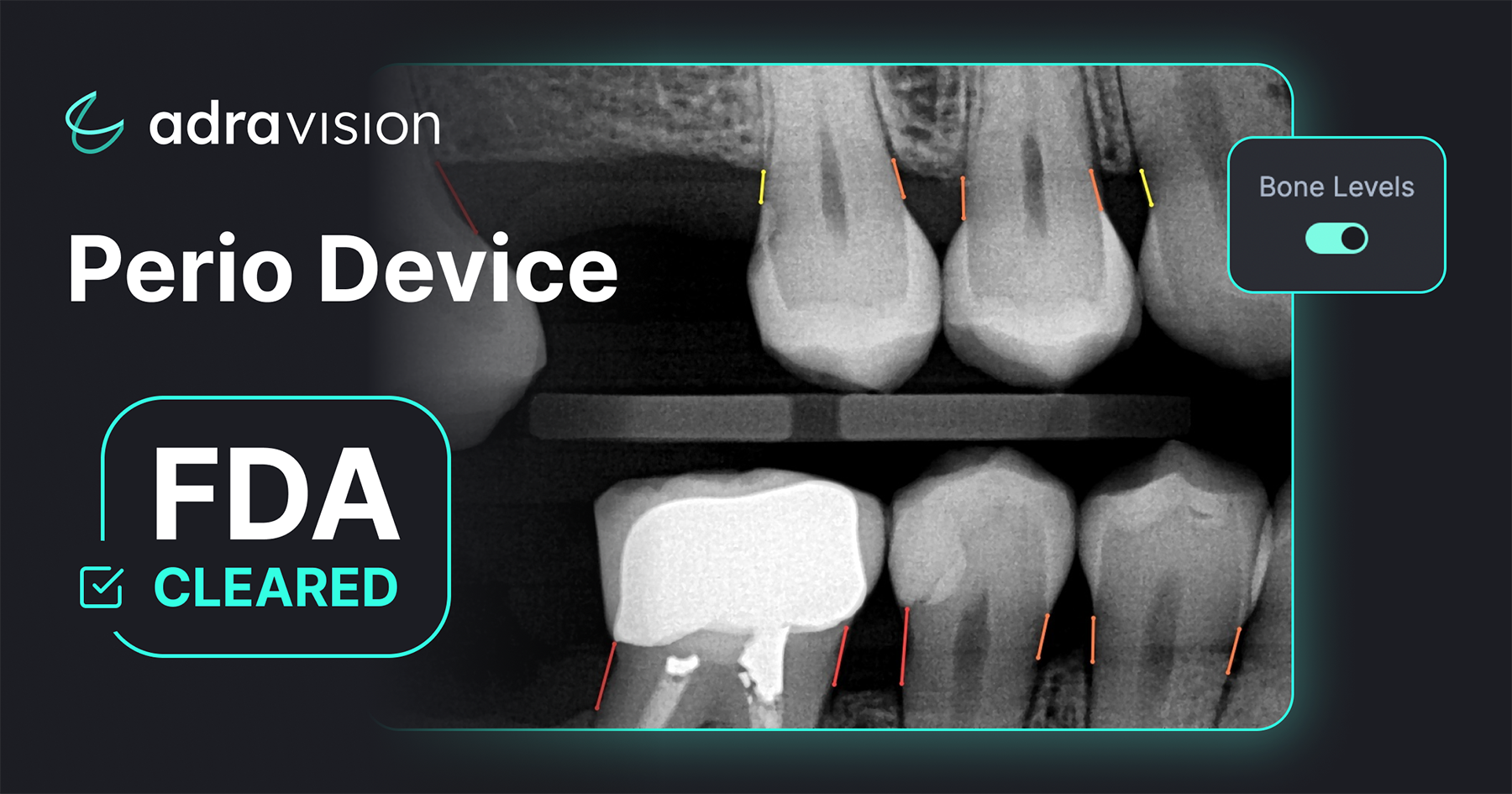
[Dental AI Software]
Adravision Perio
Dental artificial intelligence (AI) company Adravision has announced the receipt of 510(k) clearance from the U.S. Food & Drug Administration (FDA) for the commercialization of its Adravision Perio product within dental practices. This FDA clearance guarantees dental practitioners and patients the reliability and compliance of Adravision's technology with regulatory standards. Adravision’s Software as a Medical Device (SaMD) employs real-time AI to aid dentists and hygienists in assessing mesial and distal bone levels in bitewing and periapical radiographs. Periodontitis, if untreated, can lead to tooth loss, impaired chewing, and has associations with heart disease and arthritis, among other health concerns. Adravision Perio not only is designed to enhance patient comprehension of oral issues, leading to increased treatment acceptance rates, but it also streamlines the claims process for insurers, reducing fraud. It represents an innovative platform that merges the expertise of scientists, dental practitioners, and AI professionals to improve the interpretation of dental radiographs. The platform's robust Software as a Service (SaaS) tools empower clinicians to streamline their workflow, reduce time spent on interpretations and charting, and ultimately deliver optimal patient care.