Neocis Launches New Bone Reduction Feature for Yomi Robot
This new United States Food and Drug Administration-cleared bone reduction feature will allow clinicians using Yomi to save time safely and enact precise bone reduction.
Neocis Launches New Bone Reduction Feature for Yomi Robot
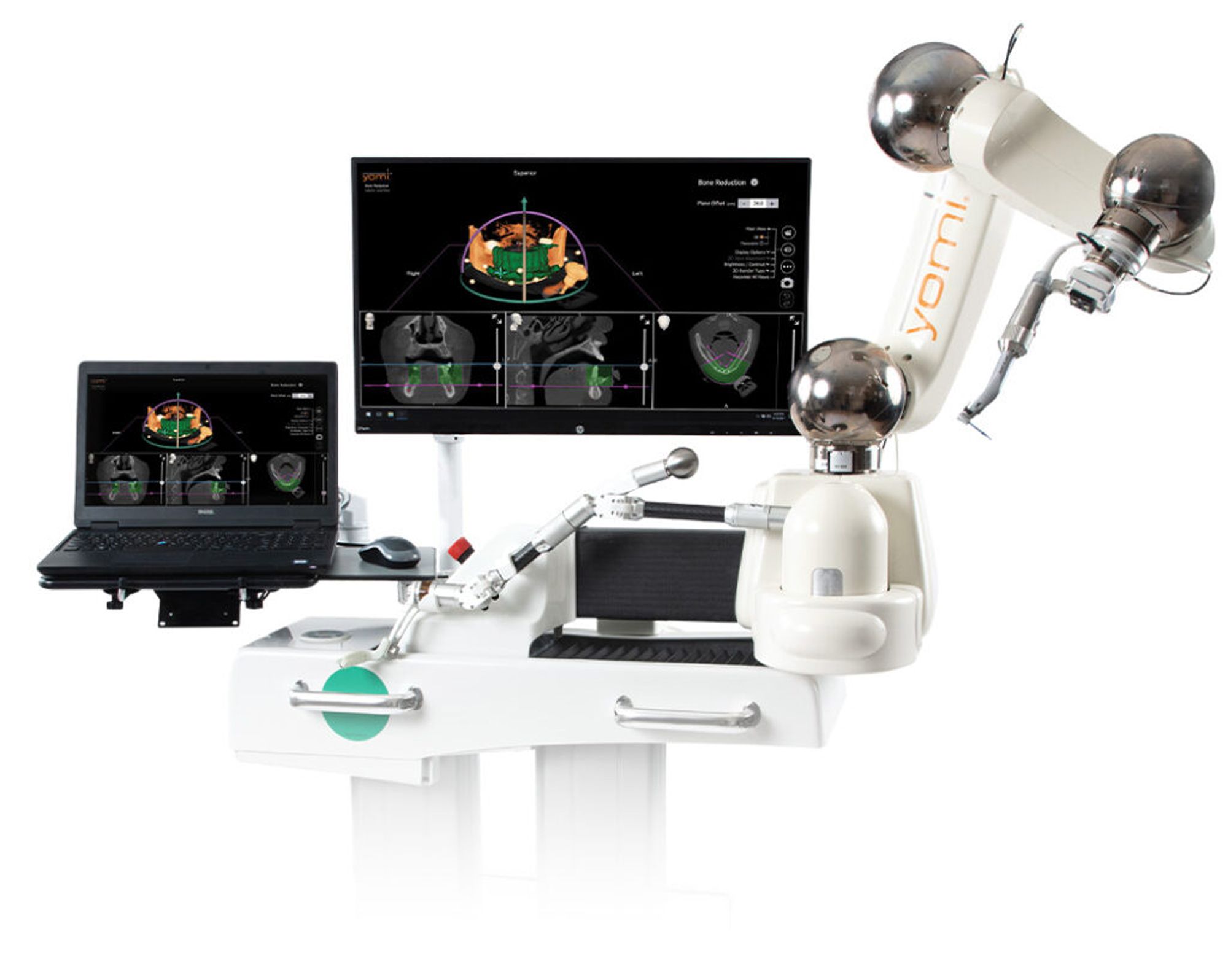
Dental robotics company Neocis has announced the commercial launch of its bone reduction planning feature for the Yomi robotic, dental surgery system. With Yomi-guided alveoloplasty, the user is physically prevented from moving the cutting bur beyond the reduction boundaries outlined in the treatment plan. Robotic assistance also provides the capability for intraoperative treatment modifications, unobstructed visualization, irrigation, and palpation of the surgical site. Surgeons receive a clear virtual reference of how much bone has been removed, providing a time-saving, safe solution, according to a press release from Neocis.
“Yomi saves me a bunch of time by allowing me to precisely plan every dimension of the reduction volume for each patient,” Yomi-user and Seattle-area oral and maxillofacial surgeon Dr Scott Clayhold says. “Then, I have the clinically flexibility to take the bur where I want, knowing that Yomi will prevent me from going beyond the boundaries I’ve set in my plan. That’s the beauty of robot-guided surgery.”
Yomi is the first and only robotic system for dental surgery that is cleared by the United States Food and Drug Administration (FDA), and along with its YomiPlan™ planning software, aid clinicians in both the planning and operative phases of dental surgery. This bone reduction platform is Yomi’s newest feature and received FDA clearance in November 2022. It has been a huge boon since its launch, per Baltimore-Washington periodontist and one of its first users, Dr Sanju Jose.
“I’ve been doing full-mouth reconstruction for a long time, and I can say that Yomi takes bone reduction to the next level,” Dr Jose says in the press release. “After just a few cases, I knew this functionality would be a game-changer for us. Every time my team uses Yomi for ridge reduction, we look at each other and say, ‘this is amazing.’”
ACTIVA BioACTIVE Bulk Flow Marks Pulpdent’s First Major Product Release in 4 Years
December 12th 2024Next-generation bulk-fill dental restorative raises the standard of care for bulk-fill procedures by providing natural remineralization support, while also overcoming current bulk-fill limitations.