Navident 3 Dental Surgery Navigation System Receives FDA Clearance
ClaroNav Inc.’s system’s increased versatility enables it to guide the drilling for implant placement in practically any jaw, as well as reduces pre-surgical preparation time and procedure costs.
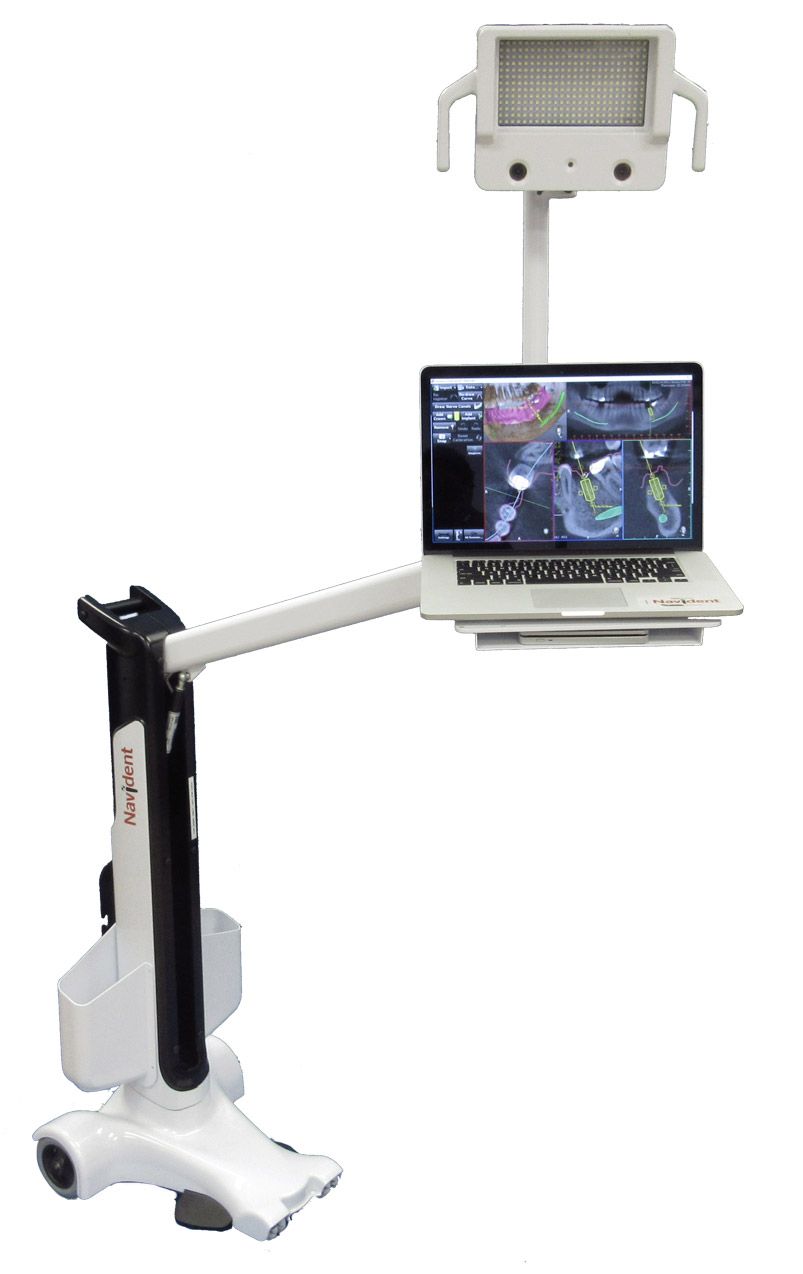
ClaroNav Inc. has received 510(k) clearance from the US Food and Drug Administration (FDA) for the 3rd generation of Navident, and the company expects this clearance to make it even more compelling for American dental surgeons to integrate Navident into their daily workflow.
This latest develop expands the earlier clearance of Navident to include guiding the treatments of fully edentulous (toothless) jaws, the use of additional CT-to-jaw registration methods, and a set of reusable accessories supporting these methods.
Navident 3 dental surgery navigation system’s increased versatility enables it to guide the drilling for implant placement in practically any jaw, as well as reduces pre-surgical preparation time and procedure costs. The innovative registration methods cleared include trace registration, touch registration, and NaviBite registration. The reusable new tools include a tracer and several types of reusable jaw trackers that enable the navigation system to track jaw motions in a minimally intrusive manner.
By widening its indications for use to include any patient with missing teeth, including the 10 percent of US population that is missing all of their teeth, this clearance will make it even more compelling for more dental surgeons to integrate the technology into their daily workflow.
“Navident, with its highly innovative Trace-and-Place and Touch-and-Place workflows, facilitates same day surgery and full arch restoration, providing the fast and efficient precision dentistry that many of these patients are looking for and can now benefit from,” says Jason Pardo, Global VP of Sales and Marketing for ClaroNav.
Since the previous FDA clearance in 2016, the company has, in response to user feedback, greatly improved Navident’s design with many unique innovations and inventions (6 US patents issued or pending since 2016).
Doron Dekel, ClaroNav’s Co-CEO, adds, “As part of the development, we have made a large investment in testing and benchmarking the upgraded system to ensure its safety and effectiveness in the wide range of clinical situations it may encounter in practice. I am pleased that, following a thorough review of our quality assurance measures, the FDA cleared our application. I am also pleased that we are able to make all these design improvements available at an incremental cost to all our American customers, even those who bought their system back in 2016.”
ClaroNav developments surgical navigation solutions, including the following products/divisions; Navident, Navient/Cranial, NaviX/OEM and the MicronTracker camera, all designed with the ultimate goal of helping practitioners and their patients live better lives.
ACTIVA BioACTIVE Bulk Flow Marks Pulpdent’s First Major Product Release in 4 Years
December 12th 2024Next-generation bulk-fill dental restorative raises the standard of care for bulk-fill procedures by providing natural remineralization support, while also overcoming current bulk-fill limitations.
ACTIVA BioACTIVE Bulk Flow Marks Pulpdent’s First Major Product Release in 4 Years
December 12th 2024Next-generation bulk-fill dental restorative raises the standard of care for bulk-fill procedures by providing natural remineralization support, while also overcoming current bulk-fill limitations.
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.