How to use cavity liners to provide better care
Why one practitioner says that TheraCal LC® from Bisco is the closest thing to an ideal material.
As dental students, most of us were indoctrinated with the calcium hydroxide philosophy. That is, we placed calcium hydroxide under all restorations. Brands such as Dycal® (Dentsply Sirona) and Life™ (Kerr) were to dentists as ubiquitous as Kleenex is to our patients. The philosophy served the profession well, until the concept was challenged.1,2
The renegades who challenged the calcium hydroxide cult met stiff resistance and, in my humble opinion, were treated as heretics.3, 4 A slew of scientific articles debated the issue of pulpal protection, some with dramatic titles (“The disastrous effects of the ‘total etch technique’ in vital pulp capping in primates”) while other studies had totally differing results.5,6 Now after almost two decades of clinical practice, literally tens of millions of human teeth in this country have had dentin etched (76 million composite restorations in one year alone, 2004), and the pulps have survived.7
There is good reason for the survival of the vast majority of dental pulps. Dentin has significant buffering capacity, as would logically be expected due to evolutionary development.8 In addition, the dentino-pulpal complex utilizes a dentino-pulpal fluid flow that helps protect the pulp and remineralizes the dentin.9 In the diseased state, the dentin caries progresses due to the extreme acidity of lactic, acetic and, interestingly, propionic acids produced by cariogenic bacteria.10
The acidic environment demineralizes the dentin but - equally importantly - stimulates the matrix metalloproteinases (MMPs 1, 2, 8 and 9) to break down the collagen matrix.11 Humans and our microbiome have co-evolved so that we have developed symbiotic relationships, including within the oral cavity. Indeed, the host and the symbiont microbiome form a holobiont virtually sharing the hologenome.12 It has been recently stated that the microbiome is even the driving force of evolution.13 As such, the dental caries process has developed a complex microbiome, not only of cariogenic bacteria but also of commensal competitive bacteria that help to control the carious progression.14 A process that is just now being appreciated.
One curious concept is that somehow a few seconds exposure of dentin to acid etch would severely injure pulpal tissue while the carious process has often exposed the very same dentin to a harsh acidic environment over a period of months (and during that same time the pulp was also challenged by bacterial toxins)15, 16 - especially as the overriding constant factor effecting pulpal vitality is now considered to be the presence of bacteria.17, 18 As in all surgical procedures, control of pathogenic bacteria is of primary importance.19
This is where both of the opposing camps were actually correct. The alkalinity of the calcium hydroxide products provided protection against pathogenic bacteria.20, 21 In addition, the presence of the calcium ions may strongly induce dentin bridge formation.22 A restoration that does not leak is always essential to survival of the pulp.23 The only issue with etching the dentin was the removal of the smear layer that would subsequently increase dentin permeability with hydrophilic adhesives.24 The increase in permeability would allow for not only bacterial invasion but also the penetration of cytotoxic resin components.25
Up next: Using an etching technique
Fortunately, we now have better adhesive agents that not only penetrate the etched dentin replacing the smear layer (which ironically contains bacteria, yet it has been advocated to leave this contaminated debris) - the adhesives provide a better layer of protection from pathogenic bacteria as the smear layer produces voids in the hybrid layer.26 Smear layers, left intact, reduce the bond strength of the dentin adhesives.27 Unfortunately, some dental products still contain cytotoxic components capable of pulpal insult.28-30 Also, hydrophilic dental adhesives degrade over time and stimulate MMP activity, leaving voids in the hybrid layer and allowing for decreased bond strength and increasing microleakage.31
Resin-modified glass ionomers play an extremely important role in clinical dentistry. The fluoride release is quite beneficial in re-mineralizing enamel and inhibiting cariogenic biofilms.32 However, they are quite acidic with a setting pH below 3 and the polyacrylic acid component actually inhibits apatite formation, a key precursor of dentin bridge formation.33-35 However, the release of fluoride from RMGIs helps enamel by creating fluorapatite, thereby reducing solubility36 - which is not, however, as much benefit for dentin that contains over 33 percent organic matter with only 45 percent calcium hydroxyapatite, making fluorapatite formation less significant.37
Fig. 1
The above notwithstanding, the overall clinical benefits of glass ionomer products cannot be discounted - especially in the caries-prone individual, and as polymerization contraction stress relief under restorations is quite significant.38 Unfortunately, cytotoxic constituents of glass ionomer products may again be detrimental to pulp vitality.39,40 Of interest is that clinical manifestations of pulpal irritation are not always indicative of histological findings, further confusing the situation.41
The ideal pulp capping or deep cavity material would be light-cured for easy placement and time control. The material would be alkaline and calcium-releasing to encourage dentin bridge formation. In addition, the material would have sufficient mechanical properties. The dental material closest to having all these properties is TheraCal LC® (BISCO), a light-cured, resin-based dicalcium and tricalcium silicate.42,43
Clinical case
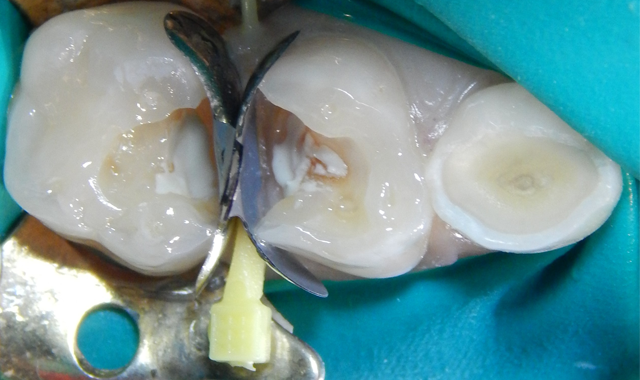
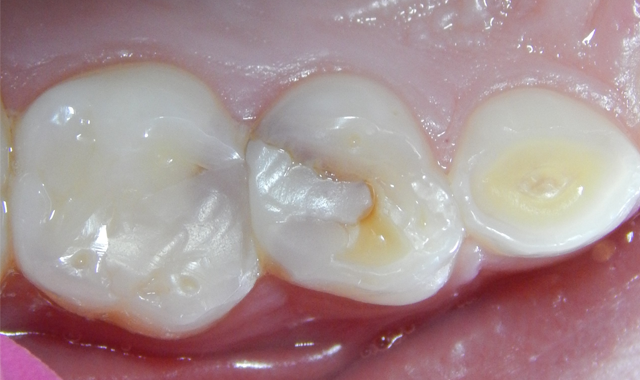
A 7-year-old male patient presented with a history (maternal report) of severe dental apprehension and extensive recurrent caries. Upon examination the patient was noted to have a strong but manageable gag reflex, some airway concerns and slight “long face syndrome.” The dentition was mixed with permanent first molars and incisors. Patient reported sensitivity with eating on the upper-right quadrant and the primary molars restorations in that quadrant presented with severe micro-leakage (Fig. 1). The patient was appointed for restorative care as soon as possible.
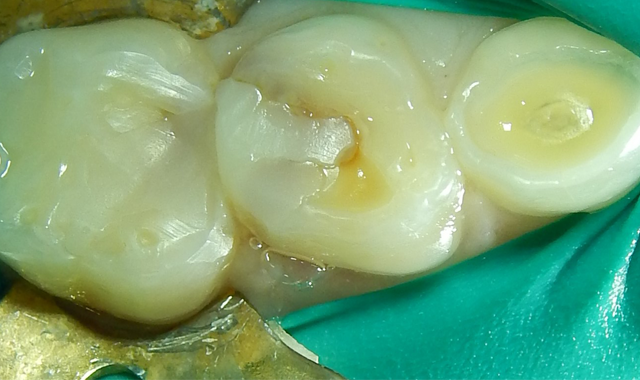
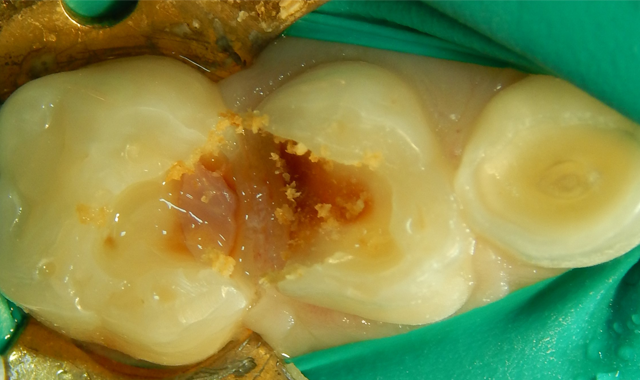
Fig. 2 Fig. 3
Upon the operative visit he remained very cooperative. Single Tooth Anesthesia® (Milestone Scientific) was utilized for local anesthesia and a rubber dam placed (Fig. 2). Removal of the previous restorations was quick, as they demonstrated no evidence of bond with tooth structure. Gross caries was present under the restorations along with obvious micro-leakage staining (Fig. 3).
Following gross carious dentin removal, the pulps were not exposed but a pinking blush was noticeable. Following debris removal by copious rinsing, the enamel margins were etched with UNI-ETCH® w/ BAC (BISCO). The dentin was etched to remove the smear layer but not widen the dentinal tubules (Figs. 4-5).

Fig. 4 Fig. 5
Leaving dentin moist (dentin should never be dried anyway) TheraCal LC is applied and light-cured. Because TheraCal LC incorporates hydrophilic monomers, it spreads better on moist dentin and adheres after light curing (Fig. 6). The alkaline pH and calcium release, very similar to ProRoot MTA (Dentsply), stimulates dentin formation and inhibits bacterial growth.44,45
The etched dentin was hybridized with ALL-BOND UNIVERSAL® adhesive (BISCO), a seventh-generation adhesive, and was restored with a resin-based dual cure (bulk fill) composite (Fig. 6). After removal of the rubber dam, the patient thanked me for making him “numb.” He claimed it was the first time he had been anesthetized for dental care. His mother was unaware that he had never received anesthesia for treatment, and had thought that he was just being “too sensitive.” The patient has returned for recare appointments and the teeth have remained asymptomatic (Fig. 7).
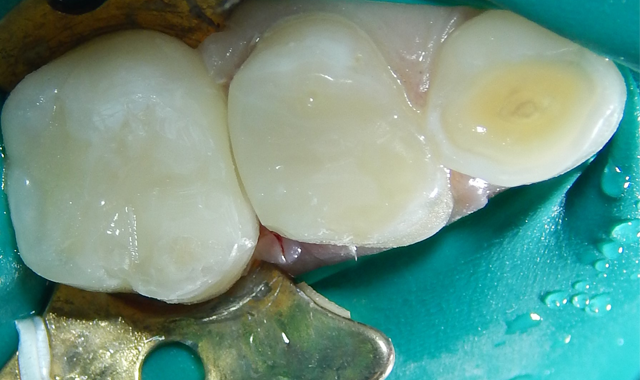
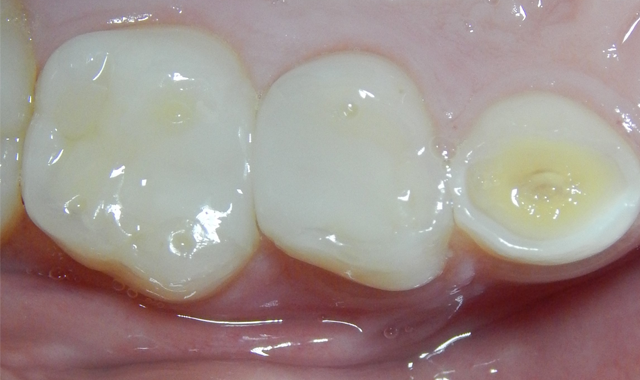
Fig. 6 Fig. 7
Proper restorative care should include local anesthesia when appropriate, proper isolation and the correct choice of dental materials. TheraCal LC is the modern replacement for older cavity liners such as Dycal and Life, which are not indicated for placement under newer restorative materials (resin-based composites) used in the contemporary dental practice.
References:
1 Fusuyama T. New Concepts in Operative Dentistry. Chicago, Ill: Quintessence Publishing Co, Inc; 1980.
2 Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substances. J Biomed Mater Res. 1982;16:265-273.
3 Kanca J. A method for bonding to tooth structure using phosphoric acid as a dentine-enamel conditioner. Quintessence Int. 1991;22:285-290.
4 Kanca J. Improved bond strength through acid etching of dentin and bonding to wet dentin surfaces. J Am Dent Assoc. 1996a;123:35-43.
5 Pameijer CH, Stanley HR. The disastrous effects of the "total etch" technique in vital pulp capping in primates. Am J Dent. 1998 Jan;11 Spec No:S45-54.
6 Hafez, A et al. “An in vivo evaluation of hemorrhage control using sodium hypochlorite and direct capping with a one- or two-component adhesive system in exposed nonhuman primate pulps.” Quintessence international 33 4 (2002): 261-72.
7 American Dental Association (2007) 2005-06 Survey of Dental Services Rendered. American Dental Association, Survey Center. Chicago, IL
8 Teaford, M, Smith, M Ferguson, M. Development, function and evolution of teeth. (Cambrige University Press, 2000).
9 Rangcharoen M., Sirimaharaj V., Wanachantararak S., Vongsavan N., Matthews B. Observations on fluid flow from exposed dentine in primary teeth: An in vitro study. Arch Oral Biol. 2017 Nov;83:312-316. doi: 10.1016/j.archoralbio.2017.08.013. Epub 2017 Aug 25.
10 Hojo S, Komatsu M, Okuda R, Takahashi N, Yamada T. Acid profiles and pH of carious dentin in active and arrested lesions. J Dent Res. 1994 Dec;73(12):1853-7.
11 Chaussain-Miller C1, Fioretti F, Goldberg M, Menashi S. The role of matrix metalloproteinases (MMPs) in human caries. J Dent Res. 2006 Jan;85(1):22-32.
12 Rosenberg E, Zilber-Rosenberg I. Microbes Drive Evolution of Animals and Plants: the Hologenome Concept. MBio. 2016 Mar 31;7(2):e01395. doi: 10.1128/mBio.01395-15.
13 Zilber-Rosenberg I, Rosenberg E. Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiol Rev. 2008 Aug;32(5):723-35. doi: 10.1111/j.1574-6976.2008.00123.x. Epub 2008 Jun 28.
14 Belda-Ferre P1, Alcaraz LD, Cabrera-Rubio R, Romero H, Simón-Soro A, Pignatelli M, Mira A The oral metagenome in health and disease. ISME J. 2012 Jan;6(1):46-56. doi: 10.1038/ismej.2011.85. Epub 2011 Jun 30.
15 Hiraishi N, Kitasako Y, Nikaido T, Foxton RM, Tagami J, Nomura S. Evaluation of active and arrested carious dentin using a pH-imaging microscope and an X-ray analytical microscope. Oper Dent. 2003 Sep-Oct;28(5):598-604.
16 Murakami K, Kitasako Y, Burrow MF, Tagami J. In vitro pH analysis of active and arrested dentinal caries in extracted human teeth using a micro pH sensor. Dent Mater J. 2006 Sep;25(3):423-9.
17 Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, Dewhirst FE, Leys EJ, Paster BJ. Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol. 2008 Apr;46(4):1407-17. doi: 10.1128/JCM.01410-07. Epub 2008 Jan 23.
18 Cooper PR, Chicca IJ, Holder MJ, Milward MR. Inflammation and Regeneration in the Dentin-pulp Complex: Net Gain or Net Loss?J Endod. 2017 Sep;43(9S):S87-S94. doi: 10.1016/j.joen.2017.06.011.
19 Tziafas D, Koliniotou-Koumpia E, Tziafa C, Papadimitriou S. Effects of a new antibacterial adhesive on the repair capacity of the pulp-dentine complex in infected teeth. Int Endod J. 2007 Jan;40(1):58-66.
20 Zancan RF, Vivan RR, Milanda Lopes MR, Weckwerth PH, de Andrade FB, Ponce JB, Duarte MA. Antimicrobial Activity and Physicochemical Properties of Calcium Hydroxide Pastes Used as Intracanal Medication. J Endod. 2016 Dec;42(12):1822-1828. doi: 10.1016/j.joen.2016.08.017. Epub 2016 Oct 21.
21 Mohammadi Z, Dummer PM. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J. 2011 Aug;44(8):697-730. doi: 10.1111/j.1365-2591.2011.01886.x. Epub 2011 May 2.
22 Farhad A, Mohammadi Z. Calcium hydroxide: a review. Int Dent J. 2005 Oct;55(5):293-301.
23 TJ Hilton. Keys to Clinical Success with Pulp Capping: A Review of the Literature. Oper Dent. 2009 Sep–Oct; 34(5): 615–625.
24 Manar M, Abu-Nawareg, Zidan A, Zhou J, Agee K, Chiba A, Tagami J, Pashley, D. Adhesive sealing of dentin surfaces in vitro: A review Am J Dent. 2015 Dec; 28(6): 321–332.
25 Dursun, E, Fron-Chabouis, H, Attal, J.-P, Raskin, A. (2016). Bisphenol A Release: Survey of the Composition of Dental Composite Resins. The Open Dentistry Journal, 10, 446–453. http://doi.org/10.2174/1874210601610010446.
26 Van Landuyt KL, De Munck J, Mine A, Cardoso MV, Peumans M, Van Meerbeek B. Filler debonding & subhybrid-layer failures in self-etch adhesives. J Dent Res. 2010 Oct;89(10):1045-50. doi: 10.1177/0022034510375285. Epub 2010 Jul 14.
27 Yoshida E, Uno S. Voids formation along the bonding interface between a smeared dentin surface and all-in-one adhesives. Dent Mater J. 2004 Dec;23(4):643-9.
28 Gupta K, Saxena P, Pant V, Pant A. (2012). Release and toxicity of dental resin composite. Toxicology International, 19(3), 225–234. http://doi.org/10.4103/0971-6580.103652
29 Oliva A, Della Ragione F, Salerno A, Riccio V,Tartaro G, Cozzolino A, D'Amatoc S, Pontonia G, Zappia V. Biocompatibility studies on glass ionomer cements by primary cultures of human osteoblasts Biomaterials Volume 17, Issue 13, July 1996, Pages 1351-1356.
30 Nicholson JW, Czarneck B. The biocompatibility of resin-modified glass-ionomer cements for dentistry Dental Materials Volume 24, Issue 12, December 2008, Pages 1702-1708
31 De Munck J, Van Meerbeek B, Yoshida Y, Inoue S, Vargas M, Suzuki K, Lambrechts P, Vanherle G Four-year water degradation of total-etch adhesives bonded to dentin J Dent Res. 2003 Feb;82(2):136-40.
32 Chau NP, Pandit S, Cai JN, Lee MH, Jeon JG. Relationship between fluoride release rate and anti-cariogenic biofilm activity of glass ionomer cements. Dent Mater. 2015 Apr;31(4):e100-8. doi: 10.1016/j.dental.2014.12.016. Epub 2015 Jan 17.
33 Ben-Amar A, Liberman R, Apatowsky U, Pilo R. (1999), pH changes of glass–ionomer lining materials at various time intervals. Journal of Oral Rehabilitation, 26: 847–852.
34 Smith DC, Ruse ND. Acidity of glass ionomer cements during setting and its relation to pulp sensitivity. J Am Dent Assoc. 1986 May;112(5):654-7.
35 Kamitakahara M, Kawashita M, Kokubo T, Nakamura T. Effect of polyacrylic acid on the apatite formation of a bioactive ceramic in a simulated body fluid: fundamental examination of the possibility of obtaining bioactive glass-ionomer cements for orthopaedic use. Biomaterials. 2001 Dec;22(23):3191-6.
36 Aoba T. The effect of fluoride on apatite structure and growth. Crit Rev Oral Biol Med. 1997;8(2):136-53.
37 Ten Cate's Oral Histology, Nanci, Elsevier, 2013, page 194
38 Cannon ML. A clinical study of the "open sandwich" technique in pediatric dental practice. J Dent Child (Chic). 2003 Jan-Apr;70(1):65-70.
39 Nascimento AB, Fontana UF, Teixeira HM, Costa CA. Biocompatibility of a resin-modified glass-ionomer cement applied as pulp capping in human teeth. Am J Dent. 2000 Feb;13(1):28-34.
40 MaÅkiewicz K, WychowaÅski P, Olkowska-Truchanowicz J, Tykarska M, CzerwiÅski M, Wilczko M, Owoc A. Uncompleted polymerization and cytotoxicity of dental restorative materials as potential health risk factors. Ann Agric Environ Med. 2017 Dec 23;24(4):618-623. doi: 10.5604/12321966.1235159. Epub 2017 May 11.
41 Mickenautsch S, Yengopala V, Banerjee A, Pulp response to resin-modified glass ionomer and calcium hydroxide cements in deep cavities: A quantitative systematic review. Dental Materials Volume 26, Issue 8, August 2010, Pages 761-770
42 Cannon M, Gerodias N, Viera A, Percinoto C, Jurado R. Primate pulpal healing after exposure and TheraCal application. J Clin Pediatr Dent. 2014 Summer;38(4):333-7.
43 Cannon M. Treating Dental Trauma Managing the esthetics and protecting pulpal vitality. Inside Dentistry June 2017 Volume 13, Issue 6.
44 Gandolfi MG, Siboni F, Prati C. Chemical-physical properties of TheraCal, a novel light-curable MTA-like material for pulp capping. Int Endod J. 2012 Jun;45(6):571-9. doi: 10.1111/j.1365-2591.2012.02013.x. Epub 2012 Mar 31.
45 Cai S, Zhang W, Tribble G, Chen W. Reactions of human dental pulp cells to capping agents in the presence or absence of bacterial exposure. J Oral Sci. 2017;59(4):621-627. doi: 10.2334/josnusd.16-0625.