How to perform tissue management for accurate crown and bridge impressions
How retraction pastes can be used to great effect in restorations.
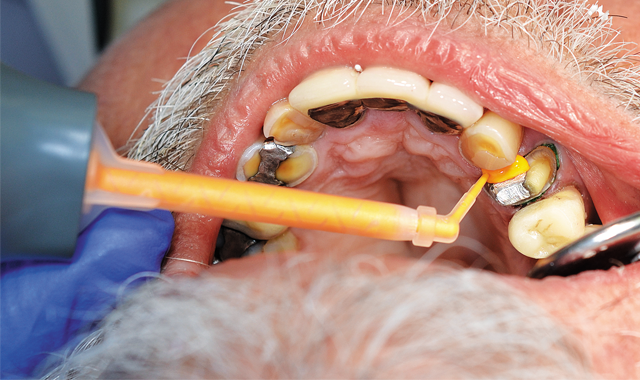
Whether practitioners record a fixed prosthodontic impression using conventional means or via digital capture, appropriate tissue management is vital.
In some cases, precise preparation of the tooth without iatrogenic damage to surrounding tissue is sufficient, while in other cases both hemostasis and gingival retraction are needed. Ideally, marginal gingiva should be healthy at the time of crown and bridge procedures.1,2 When factors such as esthetics, existing restorations or fracture dictate that finish lines of the prepared tooth are placed equi-gingival or subgingival, some form of tissue management is necessary. According to a 2015 Clinicians Report survey, 64 percent of dentists utilized gingival retraction cord and 48 percent used retraction pastes.3
There are several means of tissue retraction commonly used by dentists to create sufficient depth and width of material for crown and bridge impressions: mechanical, chemical and surgical. 3-5 Often, a combination of techniques is used.
The most common form of mechanical tissue displacement to record a conventional fixed prosthodontic impression involves the use of a gingival retraction cord. Several studies have examined the use of gingival retraction techniques by dentists, and mechanical or mechanico-chemical were most commonly utilized.4-7 Mechanical retraction using gingival retraction cord is simply the use of a string, usually made of cotton, silk or yarn wool. Products can be fabricated into configurations of knitted, braided or twisted cord of varying diameters, giving the practitioner many choices for easier placement, manipulation, absorbency and tissue retraction (e.g., Knit-Pak™ [Premier], GingiBRAID+™ [Kerr] and Z-Twist® [Gingi-Pak]).8
The use of chemical agents such as epinephrine, aluminum chloride and ferric sulfate - used alone or in combination with cords - is typical in clinical practice for fixed prosthodontics impressions. The agents can be supplied in the form of gingival retraction fluids, gels and pastes, or as a part of local anesthetic solution (e.g., epinephrine).9-10 Cords may be pre-impregnated (i.e., the chemical agent is incorporated by the manufacturer) or the chemical agent may be applied to cord by the clinician prior to placement. If chemical retraction is applied to the cord at chairside, it should be allowed to soak for approximately 20 minutes to achieve proper saturation.11
Authors have described the most desirable chemical agents to be used in gingival retraction procedures as meeting three criteria: the drug must be effective, it should not cause significant and irreversible tissue damage, and it should not produce potentially harmful systemic effects.12,13 With respect to the pharmacological effects of the active substance, the hemostatic agent aluminum chloride is most desirable because it has minimal systemic effects due to poor cell permeability, is effective for constricting blood vessels and drying tissue, will not discolor tooth structure and is the least irritating of the hemostatic agents.15-18 Although it can interact with the setting reaction of polyvinyl siloxane impression materials, this inhibitory effect is neutralized after thoroughly rinsing the area with water.18
Up next: A case study
To overcome the challenges of traditional mechanical retraction - the need for anesthesia, risk of damage to gingival and epithelial attachment, possible gingival recession, gingival inflammation and post-operative discomfort - a new class of cordless gingival retraction materials (gingival retraction pastes) has been introduced.
Generally - when clay-based to absorb moisture and coupled with an astringent - retraction pastes are designed to be placed into and around the gingival sulcus and within several minutes produce hemostasis and drying. When used with a compression cap (a cylindrical, dense cotton pellet) and direct pressure, retraction paste can also provide excellent tissue retraction. Traxodent® (Premier) is a Hemodent® Paste Retraction System that features a functionalized proprietary clay. Compared to other kaolin-based clay systems, Traxodent’s clay delivers improved ion exchange of the astringent and, due to its surface area, provides “swelling” capacity for fluid control and drying.
Traxodent contains 15 percent aluminum chloride and comes in either pre-packaged syringes utilizing bendable metal tips or unit doses with a slender plastic tip that fit into an autoclavable dispenser. Information from the manufacturer indicates that “Traxodent can be used in virtually any clinical situation in which control of bleeding is required.”19 There is essentially no learning curve to using Traxodent; the material is simply dispensed into the area around the prepared tooth (with or without retraction cord) followed by having the patient bite on a Retraction Cap (Premier).20 After two minutes, the paste is removed by thoroughly rinsing the area with water. After gentle drying, the tissue should appear free of moisture and blood and ideally prepared for the final impression.
Fig.1
Case study
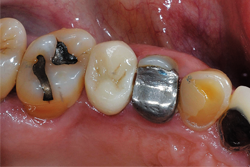
A 62-year-old male patient presented for a full-coverage restoration on tooth No. 12. The tooth had a large existing MODB amalgam build-up following successful root canal therapy. The treatment plan included a full-contour, monolithic zirconia restoration (Fig. 1).
Fig. 2

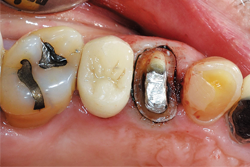
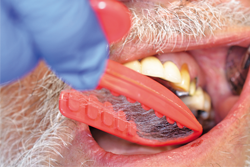
Using Solo Diamond® (Premier) Single Patient-Use diamond burs, tooth No. 12 was prepared for a zirconia restoration. At least 1 mm of occlusal and axial reduction was prepared, with a smooth 90-degree shoulder finish line ending equi-gingivally. Knit-Pak size 000 gingival retraction cord was placed atraumatically using a flat ended instrument (Figs. 2-4).
Fig. 3
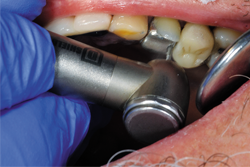
Fig. 4
Because of persistent oozing of blood and fluid around tooth No. 12, Traxodent retraction paste using a unit-dose capsule was used. After removing the individual capsule from the foil package and inserting it into the dispenser, the material was placed around the prepared tooth (Figs. 5-7).
Fig. 5
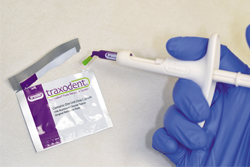
Fig. 6
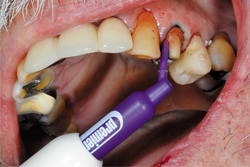
Fig. 7
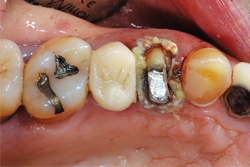
Fig. 8
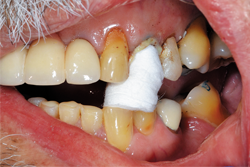
After placing the Traxodent retraction paste, a Retraction Cap was positioned so the patient could bite down and provide direct pressure to the area (Fig. 8). After approximately two minutes, the Retraction Cap was removed and the area was thoroughly rinsed with water. Hemostasis was achieved and the margins of tooth No. 12 were clearly visible (Fig. 9).
Fig. 9
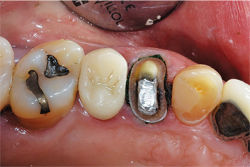
Fig. 10
The selected impression tray, in this case a T-LOC™ Triple Tray® Adhesive-Free Dual Arch Impression Tray (Premier), was tried in to verify full seating without interferences (Fig. 10). An extra-light body PVS impression material was syringed around the tooth while heavy-body PVS material was placed into the impression tray, and within the manufacturer’s intraoral working time the tray was seated (Aquasil® Ultra+ Smart Wetting® Impression Material, XLV and Heavy [Dentsply Sirona]) (Figs. 11-12).
Fig. 11
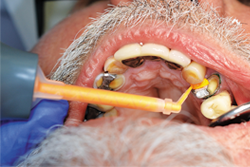
Fig. 12
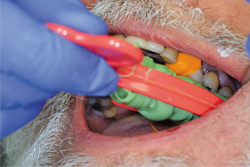
Fig. 13
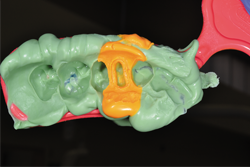
After the mouth removal time had elapsed, the impression of tooth No. 12 was removed and inspected (Fig. 13). Complete capture of the preparation details was verified, a provisional was fabricated using Luxatemp® Automix Plus (DMG America) and cemented using NexTemp® Temporary Cement (Premier), and the patient was released. He was re-appointed in three weeks for definitive cementation of the final restoration.
Conclusion
To adequately capture the details of crown and bridge impressions, the area needs to be free of blood and moisture. Typically, mechanico-chemical retraction is utilized, and when coupled with materials and techniques to consistently create astringency, better outcomes can be achieved. Traxodent retraction paste, used alone or in combination with cord and/or compression caps, provides the practitioner an armamentarium to achieve reliable tissue management.
References
1. Christensen GJ. Have fixed prosthodontic impressions become easier? J Am Dent Assoc 2003;134:1121-3.
2. Christensen GJ. The state of fixed prosthodontic impressions – room for improvement. J Am Dent Assoc 2005;136:343-6.
3. Christensen GJ. Foolproof Techniques for Optimum Impressions. Clinicians Report 2015;8(3):1-4.
4. Donovan TE, Gandara BK, Nemetz H. Review and survey of medicaments used with gingival retraction cords. J Prosthet Dent 1985;53(4):525-31.
5. Moldi A, Gala V, Puranik S, et al. Survey of impression materials and techniques in fixed partial dentures among the practitioners of India. ISRN Dentistry 2013. Available at: https://www.hindawi.com/journals/isrn/2013/430214/. Accessed November 8, 2017.
6. Azza AA, Bennani V, Chandler NP, et al. New Zealand dentists’ use of gingival retraction techniques for fixed prosthodontics and implants. N Z Dent J 2010;106(3):92-6.
7. Hansen PA, Tira DE, Barlow J. Current methods of finish-line exposure by practicing prosthodontists. J Prosthodont 1999;8(3):163-70.
8. Strassler HE, Boksman L. Tissue management, gingival retraction and hemostasis. Oral Health Journal 2011;7:35-42.
9. Nowakowska D, Panek, H. Classification of retraction materials in the aspect of biocompatibility with gingival sulcus environment. Pol J Environ Stud 2007;16:204-8.
10. Gupta G, Kumar SMV, Rao H, et al. Astringents in Dentistry: a review. Asian J Pharm Health Sci 2012;2(3):428-32.
11. Csempesz F, Vag J, Fazekas A. In vitro kinetic study of absorbency of retraction cords. J Prosthet Dent 2003;89:45-9.
12. Schillingburg HT, Hobo S, Whitsett LD, et al. Fundamentals of Fixed Prosthodontics. 3rd Ed. Carol Stream, IL: Quintessence Publishing Co. 1997. p261-7.
13. Kumbuloglu O, User A, Toksavul S, et al. Clinical evaluation of different gingival retraction cords. Quintessence Int 2007;38(2):e92-98.
14. Nowakowska D. Classification of chemical retraction agents. Protet Stomatol 2008;58:202-8.
15. Goodchild JH, Donaldson M. Local and systemic effects of mechanic-chemical retraction. Compend Contin Educ Dent 2013;34(Spec No 6):1-7.
16. Boghosian AA. Clinical and material factors in achieving the ideal impression. Available at: https://www.dentalacademyofce.com/courses/1424/pdf/clinicalandmaterialfactors.pdf. Accessed November 8, 2017.
17. Conrad HJ, Halten JR. Internalized discoloration of dentin under porcelain crowns: a clinical report. J Prosthet Dent 2009;101:153-7.
18. Tarighi P, Khoroushi M. A review on common chemical hemostatic agents in restorative dentistry. Dent Res J 2014;11(4):423-8.
19. Traxodent–Hemodent Paste Retraction System. Premier Dental Products Company. Available at: http://www.premusa.com/wp-content/uploads/2015/09/Traxodent-Brochure.pdf. Accessed November 8, 2017.
20. Using Traxodent in Clinical Practice. Inside Dentistry 2011;7(2). Available at: https://www.dentalaegis.com/id/2011/02/using-traxodent-in-clinical-practice-premier-dental. Accessed November 8, 2017.
Product Bites – November 10, 2023
November 10th 2023The weekly new products podcast from Dental Products Report is back. With a quick look at all of the newest dental product launches, Product Bites makes sure you don't miss the next innovation for your practice. This week's Product Bites podcast features new launches from Amann Girrbach, DMG, Pac-Dent, and ASI Dental Specialties. [4 Minutes]
ACTIVA BioACTIVE Bulk Flow Marks Pulpdent’s First Major Product Release in 4 Years
December 12th 2024Next-generation bulk-fill dental restorative raises the standard of care for bulk-fill procedures by providing natural remineralization support, while also overcoming current bulk-fill limitations.
Product Bites – October 27, 2023
October 27th 2023Product Bites makes sure you don't miss the next innovation for your practice. This week's Product Bites podcast features new launches from Kerr Dental, MGF, PreXion, ZimVie, Amann Girrbach, VOCO, ASI Dental Specialties, DMG, and NovoDynamics. [8 Minutes]