exocad’s exoplan 3.0 Galway Software Now FDA-Cleared for Use in the US
Release offers virtual edentulous case planning and design of surgical guides.
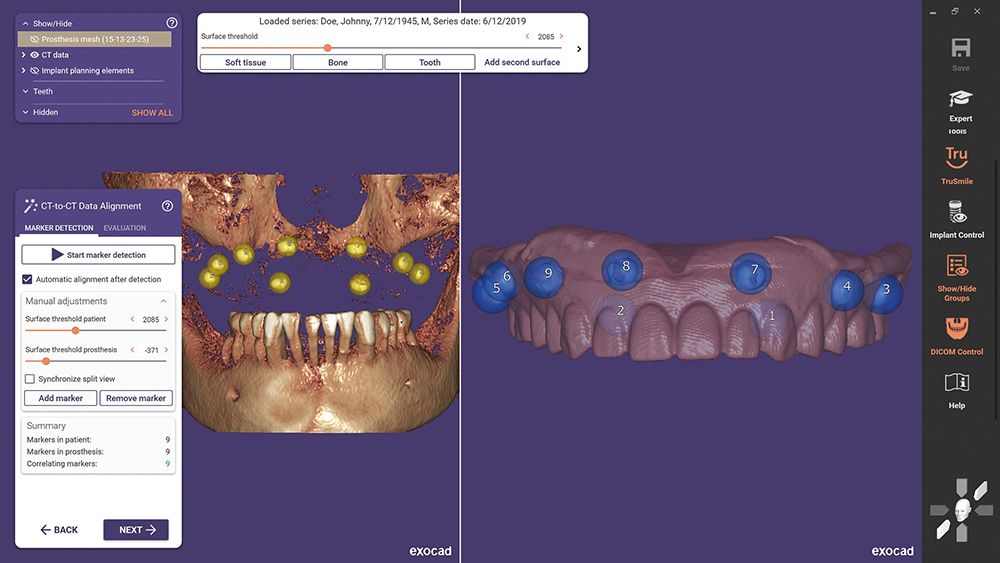
exoplan 3.0 Galway, the latest version of exocad’s implant planning software, is now U.S. Food and Drug Administration (FDA)-cleared and available for use in the United States. The new denture based surgical and fixation release supports the planning of edentulous cases, including the design of surgical guides.
exoplan 3.0 Galway is described as a powerful and efficient software package for virtual implant planning. Customized surgical guides can be designed using the Guide Creator software module and then produced in a laboratory, dental practice, or an external production center.
With 40 new features, as well as enhancements to over 60 existing functionalities, the Galway release represents a major advancement in guided surgery through improved integration with DentalCAD, exocad's dental CAD software.
"exoplan 3.0 Galway provides dental practices and laboratories with a digital implant planning workflow that offers an open collaboration and maximum flexibility," says Tillmann Steinbrecher, exocad CEO. "We are enthused about the new possibilities it presents our users to enter the world of guided surgery."
exocad, an Align Technology, Inc. company, and a leading dental CAD/CAM software provider, enables its software users to achieve predictable results in a cost- and time-efficient manner, which can help drive greater customer satisfaction, according to the manufacturer.
Key features of exoplan 3.0 Galway include:
- Planning of edentulous cases and design of the respective surgical guides, including necessary tools such as dual scan protocol, anchor pin placement, and fixation guide
- Surgical and fixation guides that can be freely designed or based on a prosthesis scan
- New tools to speed up the entire planning process
- Improved implant selection dialog
- Automatic panoramic curve detection
- More implant libraries, now with over 378 implant systems and 6,572 implants from more than 59 manufacturers
- Virtual tooth extraction on optical scans
- Clearly marked sinus cavity imaging and the ability to check if implants are intruding upon the sinus cavity
exocad names its releases after current EU "European Capitals of Culture" and selected the Irish city of Galway for this release.