DEXIS Announces Clearance of Artificial Intelligence Findings on 2D Radiographs
The company received FDA clearance for its DTX Studio Clinic, which can identify 6 types of pathological dental findings on periapical and bitewing intraoral images.
DEXIS has announced its DTX Studio™ Clinic has received US Food and Drug Administration (FDA) clearance for artificial intelligence (AI) findings on 2D intraoral x-ray images. DTX Studio Clinic now offers 6 types of pathological dental findings on periapical and bitewing intraoral images and is fully integrated into a practice’s imaging software, according to a press release from DEXIS.
DTX Studio Clinic is designed to screen dental radiographs and annotate suspected areas of interest, allowing clinicians to make informed decisions about diagnosis and treatment. These can include 6 types of pathological dental findings: caries, discrepancy at margin, root canal defects, periapical radiolucency, bone loss, and calculus. DTX Studio Clinic allows clinicians to present patients with a more comprehensive view of their diagnosis and treatment plan, which is said to build patient understanding and increase practice efficiency.
DEXIS' new DTX Studio Clinic uses AI to highlight 6 types of pathological findings on 2D radiographs, including caries.
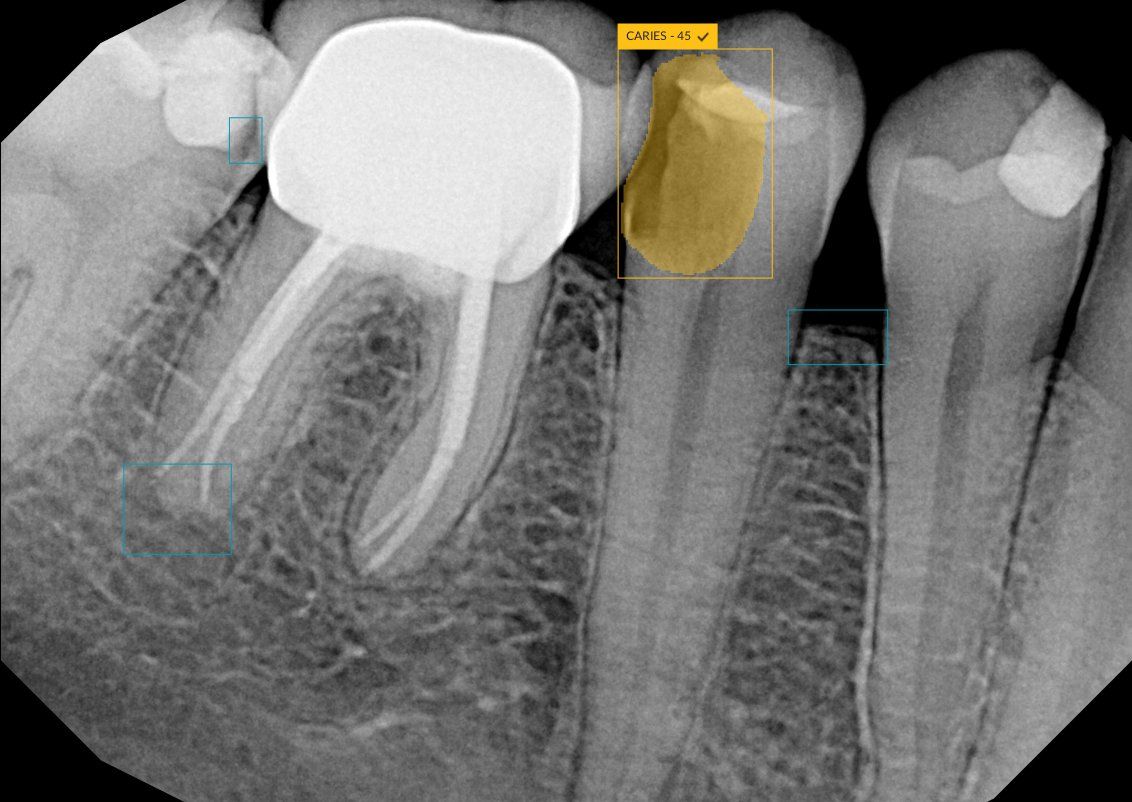
“DEXIS is empowering practices to embrace digital dentistry for improved efficiencies and predictable patient outcomes,” Amir Aghdaei, CEO of Envista, DEXIS’ parent company, said, according to the press release. “With our newest release of DTX Studio Clinic, featuring enhanced built-in AI features like the pathological dental findings, practices now have an enhanced solution for their digital workflow — from diagnosis to treatment planning to delivery of care.”
Clinicians interested to try DTX Studio Clinic AI 2D dental findings can sign up on the DEXIS website.
ACTIVA BioACTIVE Bulk Flow Marks Pulpdent’s First Major Product Release in 4 Years
December 12th 2024Next-generation bulk-fill dental restorative raises the standard of care for bulk-fill procedures by providing natural remineralization support, while also overcoming current bulk-fill limitations.