DESS Blanks and Ti-Bases Make Dental Labs FDA-compliant
The DESS Digital Dentistry workflow and products are designed in such a way to help dental labs and milling centers become compliant with FDA regulations.
DESS Blanks and Ti-Bases Make Dental Labs FDA-compliant. Image: © DESS
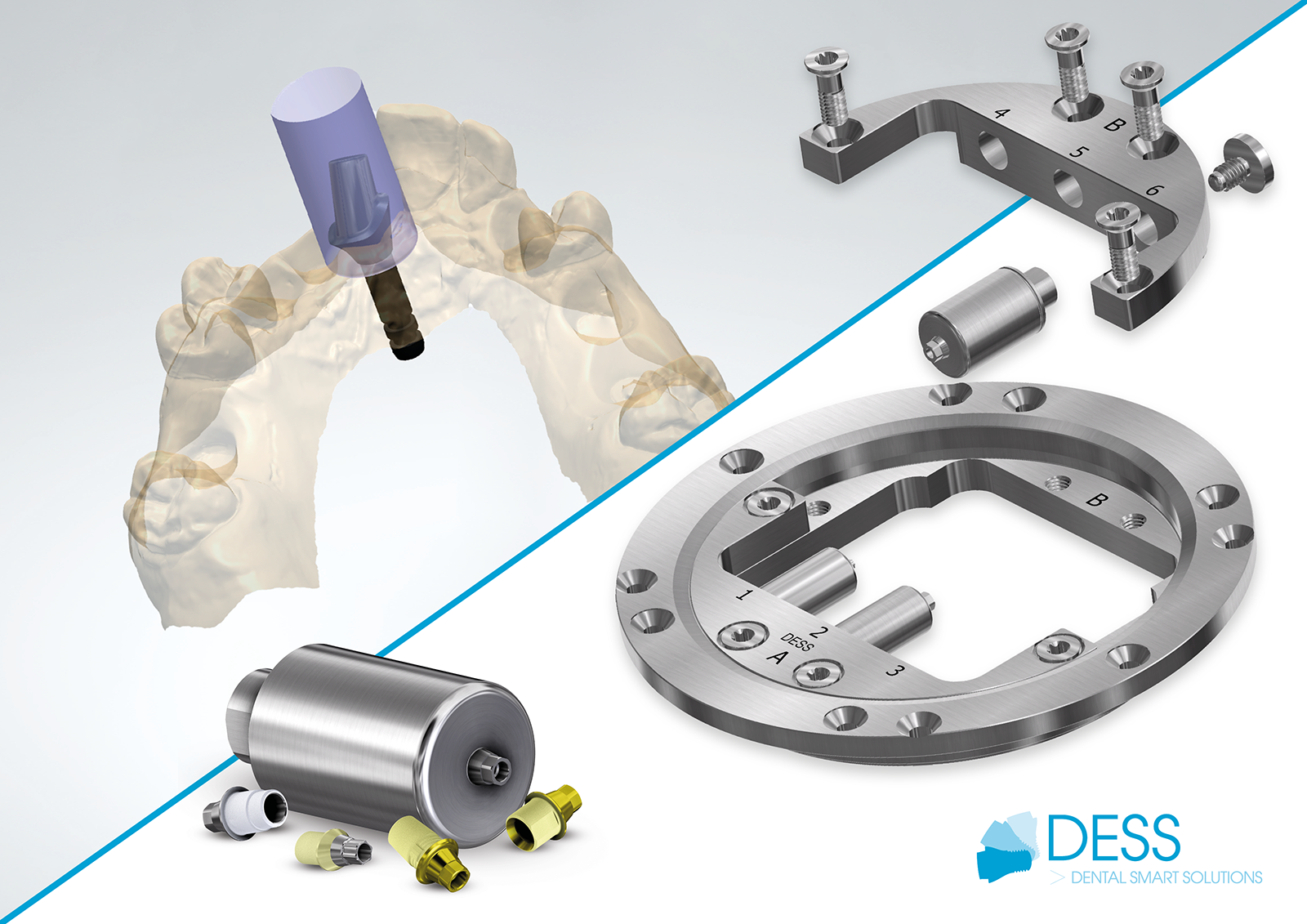
DESS® Blanks and Ti-Bases are now PNP FDA accredited, meaning dental labs can now become FDA-compliant without being FDA-registered. Because of the FDA’s evolving regulatory requirements for CAD/CAM automation processes, the 510(k) requirements for Ti blanks and Ti bases in dental implant abutments have been modified. These abutments now fall under Product Code NHA, meaning dental labs need to become “validated milling centers” and enact manufacturing procedures in line with Quality System regulations.
This all leads to laboratories needing to register with the FDA and submit to FDA audits, which may cause concern for non-FDA accredited laboratories. To ameliorate this, DESS’s new Digital Dentistry Workflow will allow these non-accredited labs to remain in operations through the use of DESS Digital Dentistry Workflow products. The DESS FDA 510(k) certification guarantees that any dental lab using this workflow is compliant with FDA and health and safety guidelines.
The following products can be used without the need for FDA certification:
- DESS Ti-Base
- DESS AURUMBase
- DESS ELLIPTIBase
- DESS C-Base
- DESS Pre-milled blanks
The DESS Digital Dentistry Workflow integrates these systems:
- 18 Implant system compatibilities
- Scan files
- CAD/CAM software
- Ceramic material
- Titanium material
- Milling machine
- Associated tooling and accessories