Dentsply Sirona, Panthera Dental Collaborating on Custom-Made Sleep Devices
With this fully digital workflow for custom-made sleep devices, dentists can now use Primescan in a new area of application: Obstructive Sleep Apnea and snoring.

Dentists helping their patients with sleeping issues including snoring has become another integral form of treatment designed to improve the health and well-being of dental patients. The collaboration between Dentsply Sirona and Panthera Dental—announced today—means dentists can now use the precise scans from Dentsply Sirona's intraoral scanner Primescan in a validated workflow as a basis to create a custom-made Digital Sleep Apnea Device (D-SAD).
Launched in 2019, Primescan is designed to enable high-precision digital impressions with outstanding imagery. The intraoral scanner can be used for patient consultation, treatment planning in implant dentistry and orthodontics, and for restorative treatments.
The quality of the intraoral scan data delivers reliable data for the high-quality custom-made D-SAD Mandibular Advancement Device (MAD), indicated for people suffering from Obstructive Sleep Apnea (OSA) and snoring.
Three steps to D-SAD: scan, send, and treat
With Dentsply Sirona’s Primescan, a complete and accurate full-arch scan is possible in as little as a minute to give the dentist immediate control of a 3D model. The optimal accuracy and reliable quality of Primescan delivers reliable data for the Panthera Dental D-SAD custom-made sleep device. This saves time and nearly eliminates the necessity for adjustments during patient visits.
The data transfer to Panthera Dental is fast and secure: for North America, Germany, France, Belgium, Netherlands, Luxembourg, United Kingdom, Australia, and New Zealand the data will be transferred securely via the Dentsply Sirona Connect Case Center. For other Primescan and Panthera Dental countries, the data can be sent via STL to Panthera Dental through their user-friendly web portal (regulations per country and availability may vary). With one of the next software versions, there will be a full integration in the Connect Case Center for all Primescan and Panthera Dental-validated countries, according to today’s press release.
How a Mandibular Advancement Device (MAD) works to overcome snoring and Obstructive Sleep Apnea (OSA).
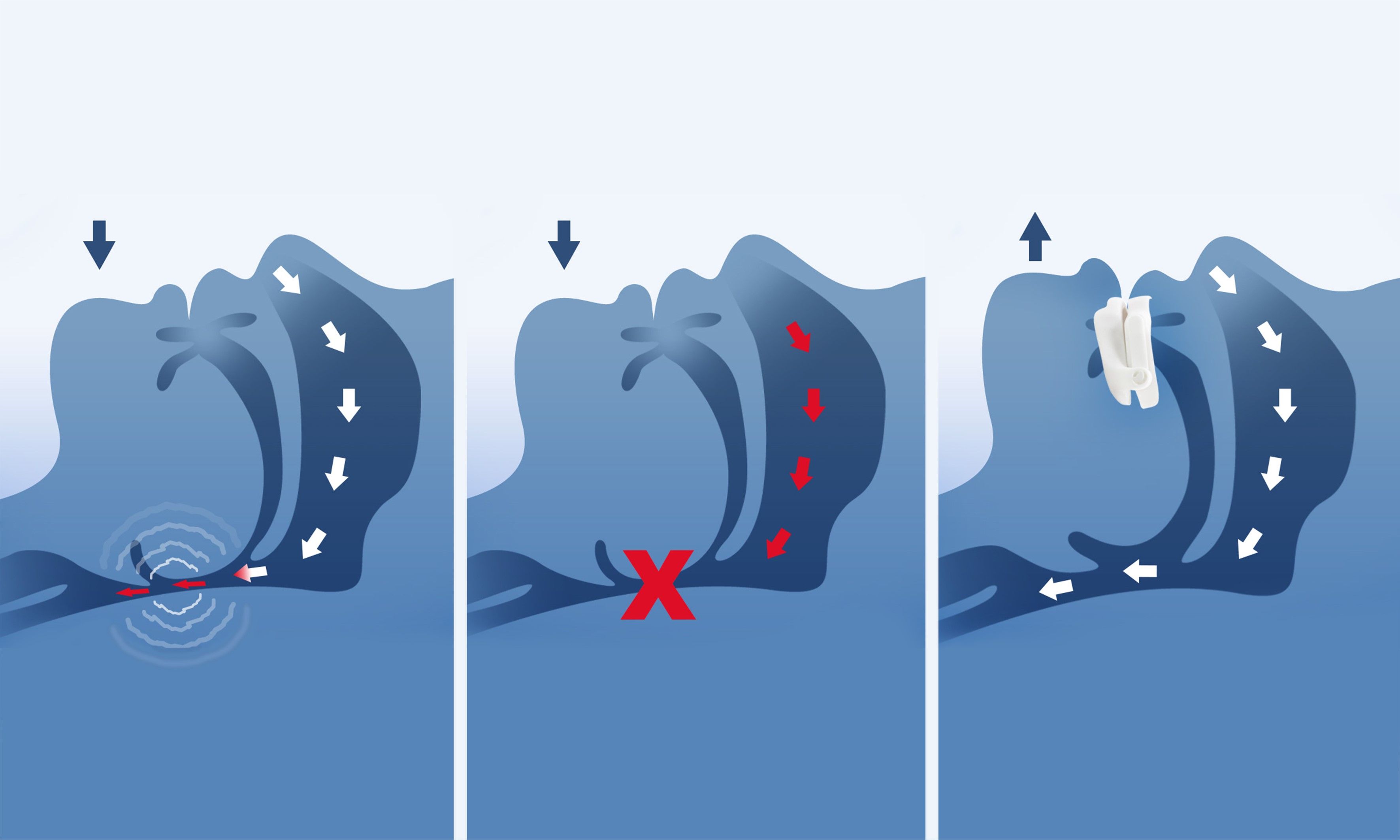
One advantage is that besides Primescan, no additional equipment is needed. After scanning the patient’s complete jaw and the bite registration for the protrusion, the scan data can be sent directly to Panthera Dental, either using the Connect Case Center or via STL-file. Within 15 days, the D-SAD will be designed and manufactured by Panthera Dental’s proprietary CAD/CAM process in their 4.0 manufacturing plant using numerous digital, robotic, and automated technologies. It is then shipped back to the dental professional via a multinational express delivery service. The dentist receives a custom-made sleep device and can start treatment immediately.
The individualized and fast result distinguishes the dental practice in its treatment of snoring and sleep apnea and allows for happier, healthier patients with improved quality of life.
Fast, accurate solution for OSA and snoring
Physical and psychological problems that sleep apnea can cause should not be underestimated. If sleep is constantly disturbed, this can have serious effects on mental and physical health. Currently, three main treatment solutions are recommended by experts: lifestyle modifications (weight loss and exercise), Continuous Positive Airway Pressure (CPAP), and Mandibular Advancement Devices (MAD). However, sleep masks (CPAP) have considerable compliance issues with 50% and 83% of patients being non-compliant after 6 months and 5 years, respectively.1,2 Patient compliance of Mandibular Advancement Devices (MAD) remains 86% after over 3 years of use.3 Thanks to Panthera Dental’s D-SAD, patients with OSA, and those who refuse or are non-compliant to CPAP therapy, have a comfortable and effective treatment option available.
Panthera Dental’s D-SAD is made out of 100% medical-grade biocompatible type-12 polyamide nylon. This cutting-edge material is light, smooth, strong yet flexible, very durable, and resistant to bruxism. A wide range of plateaus and bands are available and allow for over 300 standard design combinations as well as maximized tongue space. Additional customization is possible, and the D-SAD can be adapted to every possible patient morphology, from basic to the most complex. It is a uniquely simple and easy to care for dental appliance.
D-SAD can be used to treat sleep apnea and snoring.

The D-SAD’s unique titration mechanism uses interchangeable rods made of the same biocompatible polymer as the device, designed to not elongate and resist to bruxism. The length of the rods varies from 16 to 34 mm and can be adjusted in 0.5 mm increments for an accurate and customized advancement. Moreover, they do not disengage during sleep thanks to a patented connection system and they are easily replaced.
The Panthera D-SAD received FDA 510(k) clearance for snoring and obstructive sleep apnea has the CE mark and complies with the Canadian Medical Devices Regulations.