A composite technology to prevent secondary caries
How a new line of materials can inhibit bacterial growth without the use of chemicals.

Secondary caries is a significant cause of premature restoration failure and is a concern for both patients and dentists. That’s why I was so excited to hear about a long-lasting technology that inhibits bacterial growth and biofilm formation without the use of chemicals and without affecting the natural flora of the mouth. I wanted to learn all about it, so I sat down with the founder of Nobio, Dr. Ervin Weiss, and the company’s CEO, Yoram Ashery, to find out more.
There have been multiple attempts to incorporate antimicrobials into composite materials, so far with limited clinical impact. Why is that?
The incorporation of fluorides, silvers or any other antimicrobials relies on the active agent being released out from the composite to affect the contaminated surroundings. That release causes the concentration of the antimicrobial material to degrade in a relatively short time frame. So, there’s a limited time that existing additives in composites are active until the release is exhausted and the effect fades out.
More from the author: The AI revolution that's coming to dentistry
Another factor is that composites are technique sensitive. In certain areas of the oral cavity, it’s difficult to control moisture from saliva and gingival fluids during the procedure, and that lack of control can lead to secondary caries. For example, placing composites in a deep proximal box can lead to gaps in the subgingival or interproximal margins due to incomplete curing or other preparation challenges, increasing the potential for microleakage.
Tell me about your research into next-generation antimicrobial technologies.
An illustration of the Nobio particles permanently embedded in a material. Each particle is covered with a high concentration of anti-microbial structures that attract and destroy bacteria.

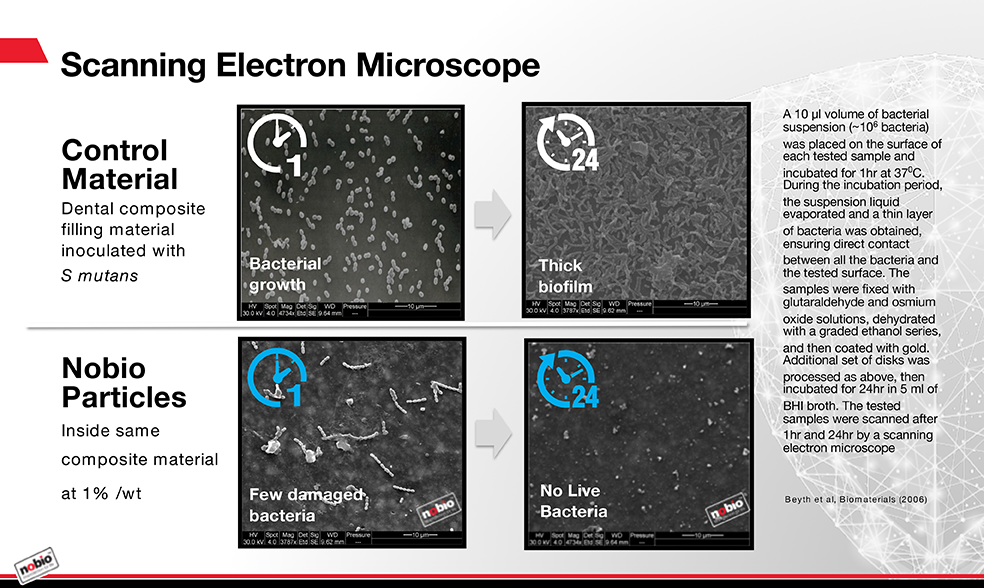
We’ve been working with The Hebrew University - Hadassah School of Dental Medicine to develop some very promising materials. Using micro- and nanoparticle technology, we’ve demonstrated an antimicrobial additive that’s nonsoluble. These particles can be mixed into the composite like a filler, along with the resin, during manufacturing and kill bacteria on contact. The additive has very strong antibacterial activity, even when it represents only 1 percent of the total mass of the composite.
Unlike current products that release chemicals to kill microbes, this technology depends on direct contact. The duration of activity is potentially unlimited, as there’s no degradation of the material. Composites incorporating these particles have been tested in the lab under accelerated aging conditions that equate to several years in natural conditions. There was no observable decline in the antibacterial activity. The material doesn’t lose mass, deteriorate or exchange with anything else in the oral environment.
An insoluble antimicrobial additive for composites seems to be a promising approach to delay or prevent secondary caries. Are there other benefits?
There are several important benefits of this next-generation approach to developing antimicrobial restoration materials. Only those bacteria that come in direct contact with a man-made surface such as a restoration are destroyed, while bacteria on natural tissue aren’t affected. Preserving the natural, healthy flora of the oral cavity is a key advantage of using an agent that’s nonsoluble and nonleaching.
Composites, bonding agents and cements incorporating these antimicrobial particles will logically be less technique sensitive or more forgiving, particularly for clinically challenging restoration sites. Because this novel antimicrobial technology kills bacteria by contact, microbes that lead to secondary caries will not survive in the micro-gap between the composite and the natural tooth.
The weakest link of all composites and bonding system combinations is at the gingival margin of the Class V or Class II restorations, where the dentin and the composite interface. Even when etched, primed and bonded properly, these marginal areas may debond over time, allowing microleakage and bacterial penetration. When an antimicrobial agent is present, penetration is less of a problem because the bacteria are going to be killed whenever they start to penetrate this micro-gap.
Preserving esthetics is another area where incorporating this technology may have an advantage. Clinical observations show that composite restorations accumulate more bacteria on their surface than the tooth surface or other restorations. In some patients, the composite changes color and becomes rough over time, losing its shine.
Trending article: 10 ways to simplify pediatric dentistry
That was an enigma for many years. In vitro studies have demonstrated that composites become rough when they accumulate bacteria on their surface, as the bacteria are capable of using the resin in the composite as a growth medium. By contrast, composites containing Nobio particles did not show this degradation and increased roughness, even under continuous bacterial challenge.
Check out this video to learn more about Nobio and antimicrobial composite materials.
What other dental applications might there be for this antimicrobial technology?
When an antimicrobial agent is present, penetration is less of a problem because bacteria are going to be killed.
The material could have positive impacts in both orthodontics and endodontics. Braces create a physical obstacle to maintaining good oral hygiene because bacteria accumulate much easier on a rough surface. If we put our technology into orthodontic cement, it may kill the bacteria that are capable of causing an acid attack and a white spot lesion around braces. The technology can also potentially be incorporated into plastic aligners and retainers, which accumulate bacteria that cause discoloration and odor.
In endodontics, researchers have reported that it’s impossible to completely eliminate bacteria from infected root canals. Regardless of a flawless technique, ideal preparation and sufficient irrigation, the bacteria are still left alive in the canal space. In addition, over time, nearly every tooth with root canal treatment eventually leaks fluid from the oral cavity through the root canal into the periapical tissue. When this happens, it’s only a question of time before the bacteria from the oral cavity will reach the apex of the tooth.
How long will it be before this technology is widely available to the dental community?
A full line of restoratives, including a universal, a flowable and a bulk fill composite, as well as a bonding agent containing the antimicrobial technology, has been developed. These products are slated for submission to the FDA in the next months. To learn more and get the latest in product development, visit nobio.com.
References:
1. Kesler Shvero D, I Abramovitz, N Zaltsman, M Perez Davidi, EI Weiss, N Beyth (2013). Towards antibacterial endodontic sealers using quaternary ammonium nanoparticles. Int Endod J.;46(8):747-54.
2. Beyth N, Kesler Shvero D, Zaltsman N, Houri-Haddad Y, Abramovitz I, et al. (2013) Rapid Kill-Novel Endodontic Sealer and Enterococcus faecalis. PLoS ONE 8(11): e78586. doi:10.1371/journal.
ACTIVA BioACTIVE Bulk Flow Marks Pulpdent’s First Major Product Release in 4 Years
December 12th 2024Next-generation bulk-fill dental restorative raises the standard of care for bulk-fill procedures by providing natural remineralization support, while also overcoming current bulk-fill limitations.