Calcivis Set to Introduce New Bioluminescent Dental Imaging System in the U.S.
Following the successful attainment of the final stage FDA Pre-Market Approval (PMA), the company plans to roll out new device at Yankee Dental Meeting.
Calcivis Set to Introduce New Bioluminescent Dental Imaging System in the U.S. | Image Credit: © Calcivis
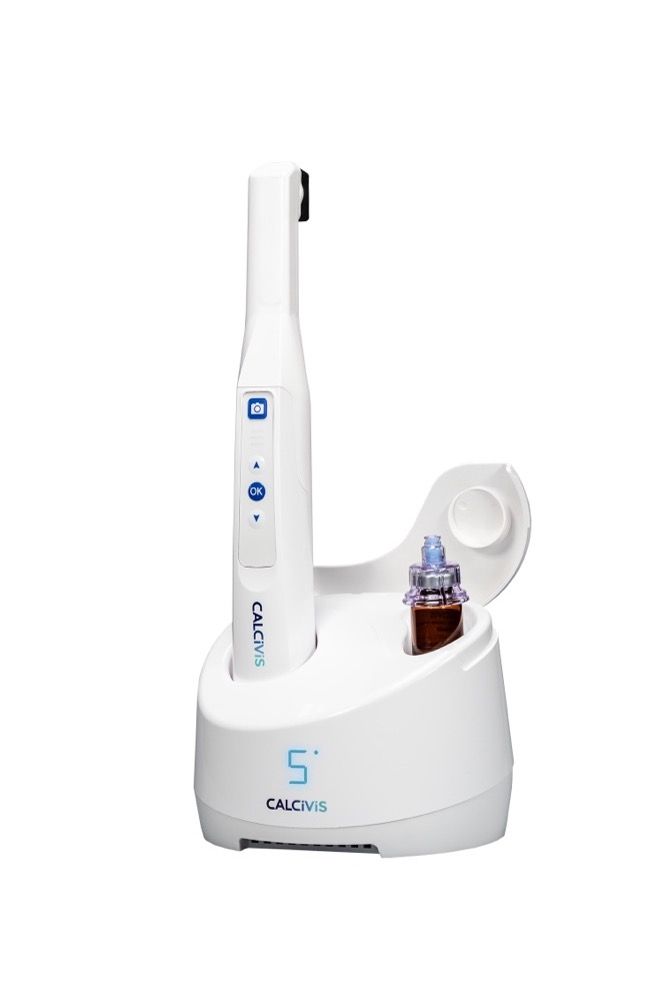
Scottish company Calcivis® is unveiling its innovative preventive dental technology in the U.S. Having submitted a Food and Drug Administration (FDA) Pre-Market Approval supplement to the FDA for improvements to its imaging system last year, the newly approved ergonomic, wireless, handheld imaging device is now ready for commercialization.
In line with the company's anticipated growth in the American market, Calcivis has established its U.S. headquarters in Milton, Massachusetts, and has bolstered its commercial team with the appointment of Ronald Frezel as vice president of sales and marketing and Jill McGregor as chief financial officer.
Both executives bring extensive experience in the dental and medical device space. Frezel, with over 20 years of experience, has played a key role in introducing dental products to the U.S. and European markets. McGregor has led financial management for multiple early-stage companies in the United Kingdom and the United States. The U.S. rollout will commence with a limited release launch in the greater Boston area in early 2024, according to Calcivis.
“With our U.S. commercial team in place and a very user-friendly, ready for market device, we are confident that the Calcivis Imaging System will significantly improve patient care and enable restorative dentistry to move to a more preventive approach,” Adam Christie, CEO at Calcivis, says in a press release.
Frezel adds in the press release: “We are excited to bring the Calcivis Imaging System to market and are planning for our launch at the Yankee Dental Meeting, to be held in Boston from January 25-27, 2024. For the first time dental professionals and their patients will be able to visualize active demineralization due to carious lesions in real time, chairside.”
The Calcivis Imaging System is poised to revolutionize caries management through early detection, allowing for a more preventive treatment model. The capability to visualize active demineralization, an early indicator of decay, in real time provides crucial insights into whether a carious lesion is likely to progress and requires treatment.
The imaging system utilizes a patented photoprotein that, in the presence of free calcium ions released from an actively decaying tooth surface, produces a brief, low-level light flash. An integrated intraoral sensor within the Calcivis imaging device promptly detects the luminescence and presents clinicians with a chairside demineralization map. The Calcivis Imaging System is safe and effective for use in adults and children aged 6 years or older and can be applied to all accessible coronal tooth surfaces, the company states.
Founded in 2012, Calcivis has secured over £18 million in funding [about US$ 22,861,000], with investors including Archangels, the world’s longest-running business angel investment syndicate, and Scottish Enterprise, Scotland’s national economic development agency.
Calcivis has pioneered a novel imaging technology that allows clinicians to visualize early signs of active decay, enabling intervention before the development of a cavity that would require drilling and filling.
Additional information can be found at calcivis.com.
ACTIVA BioACTIVE Bulk Flow Marks Pulpdent’s First Major Product Release in 4 Years
December 12th 2024Next-generation bulk-fill dental restorative raises the standard of care for bulk-fill procedures by providing natural remineralization support, while also overcoming current bulk-fill limitations.