Calcivis Caries Detection Technology Approved to Begin United States Expansion
This decay detection technology uses luminescence to visualize caries for clinicians and is now planning to expand to the United States dental market.
Calcivis Caries Detection Technology Approved to Begin United States Expansion
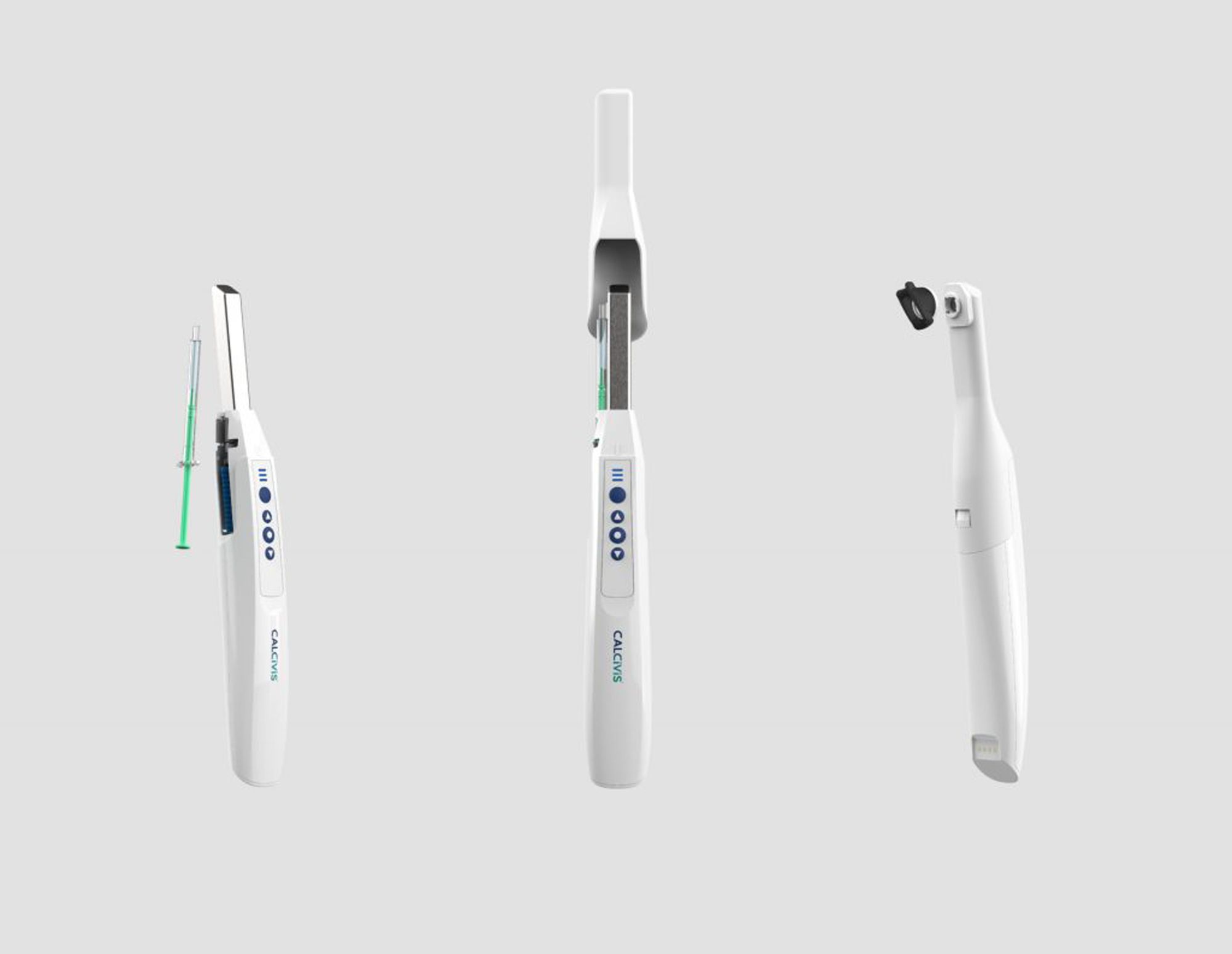
Scottish dental imaging company Calcivis has announced that is has received pre-market approval (PMA) from the United States Food and Drug Administration (FDA) for its Calcivis Imaging System. The Calcivis Imaging System applies a patented photoprotein that creates a low-level light flash using releasing calcium ions from the decay on the tooth surface. An intraoral sensor inside the Imaging System detects the light flash, and visualizes the decay for the clinician.
With its PMA, the Calcivis Imaging System now has the ability to expand into the United States, a goal that the company has in sight, according to CEO Adam Christie.
“It’s been a long process with delays caused by Covid restrictions, so we’re very pleased to have achieved PMA status,” Christie says in a press release from the company. “We have an ambitious growth plan for the highly developed U.S. developed dental market which, with our FDA accreditation, we can now capitalize on.”