Buzzy Pain Relief Device Cleared by United States Food and Drug Administration
This bumblebee-shaped device can now be used in dental practices to provide patients a needle-free, opioid-free option to reduce anxiety and pain.
Buzzy Pain Relief Device Cleared by United States Food and Drug Administration. Image: © Pain Care Labs
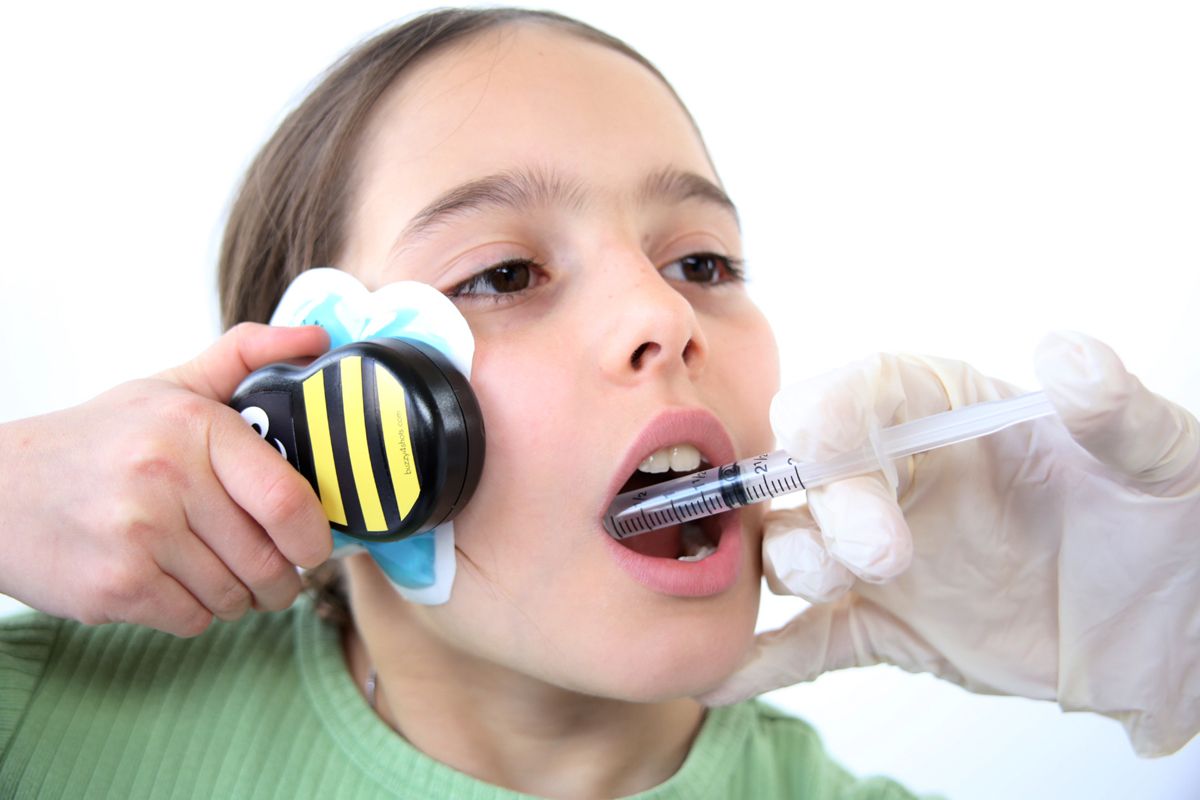
The United States Food and Drug Administration (FDA) has given a medical device called Buzzy from Pain Care Labs, 510(k) clearance for use during and after dental visits. This handheld device, which takes the shape of a bumblebee, is designed to mechanically stimulate the jaw for pain relief when used in tandem with an ice pack. This mechanical stimulation, formally called M-Stim®, is said to use a frequency that works with the cold in the ice pack to enable the brain to filter out pain. Buzzy is designed to reduce pain in injections, more so than gels or sprays, according to a press release from the company. This helps eliminate common fears regarding pain at the dental practice, according to Dr Rajiv Patel at Endodontic Excellence in Flower Mound, Texas.
“As an endodontist, I see many patients who are anxious about injections,” Dr Patels says in the press release. “I have used Buzzy for years and patients really like the experience. The injections go smoothly, and our team saves time.”
Patients place Buzzy and an ice pack on their jaw or cheek, jiggling the device to enable the M-Stim technology to fight against pain. It can be used after any dental treatment and is particularly effective around injection sites. This is a huge boon for patients who experience needle anxiety, which has trended upward since the COVID-19 pandemic, per Pain Care Labs CEO and Chief Medical Officer Amy Baxter, MD.
“The new FDA 510(k) clearance for dental procedures marks a pivotal moment in our mission to eliminate needless pain, reduce opioid use, and overcome fear in medical settings,” Dr Baxter says in the press release. “We are thrilled to officially expand the application of our pain reduction technology to the dental field, providing patients with a relaxed, pleasant experience during their dental visits. Buzzy could transform the way people think about the dentist.”
ACTIVA BioACTIVE Bulk Flow Marks Pulpdent’s First Major Product Release in 4 Years
December 12th 2024Next-generation bulk-fill dental restorative raises the standard of care for bulk-fill procedures by providing natural remineralization support, while also overcoming current bulk-fill limitations.