Basic Guidelines for Dental Water Quality
While it’s difficult to completely eradicate waterborne pathogens from healthcare water systems, infections caused by these microorganisms are preventable.
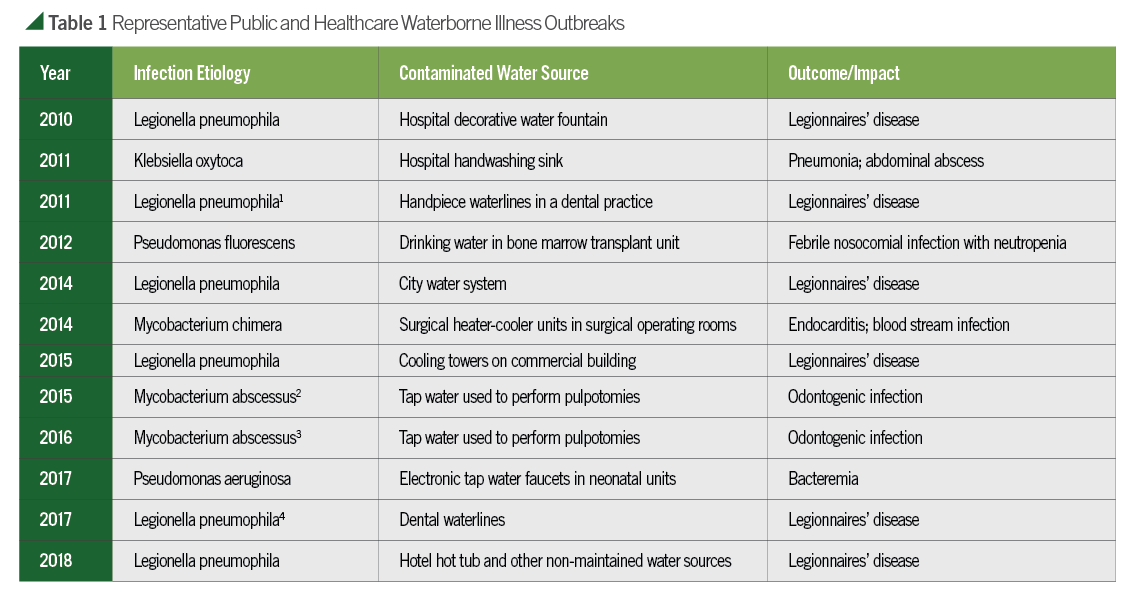
News reports and clinical articles documenting microbial infections traced back to contaminated public and healthcare water sources have increased at an alarming rate within the last decade (Table 1). Four incidents in dental practices have also been reported during this period.1-4 Localized cutaneous infections, bacteremias, and even disseminated, life-threatening diseases have been diagnosed, especially when they develop in immune compromised and/or critically ill patients.
Clinicians are asked to devote more attention to understanding possible treatment sequelae for the increasing number of compromised patients, while also minimizing microbial exposure for all patients using established infection prevention practices.
Efforts to prevent contamination of water sources in healthcare settings have improved at a rapid pace. With specific regard to dentistry, colonization of dental treatment water can be greatly reduced by appropriate application of a number of direct and indirect antimicrobial technologies. Progress aimed at addressing this emerging issue has led to the development and availability of a number of products for use in dental settings. Unfortunately, clinicians may become confused by competing and conflicting efficacy claims. In such cases, resultant dental unit waterline (DUWL) infection control procedures can be compromised and/or ineffective due to lack of following proper instructions.
In order to maximize infection prevention efforts, dental professionals should be aware of a few basic guidelines when selecting and using products.
- EPA-registration: Most dental unit waterline treatment products are EPA-registered to reduce microbial contamination. Product manufacturers are required to submit appropriate test data to validate and ensure efficacy of their product(s). If used according to the manufacturer’s instructions, EPA-registered products will perform according to its approved label.
- Manufacturers’ instructions: Follow the manufacturer’s instructions for use (IFUs) in order for the product to perform as indicated on the label. Compliance with IFUs is a major factor contributing to success, as well as a common reason for failure of a practice’s dental water infection prevention efforts. The efficacy of products is dependent on properly following IFUs. For example, ongoing protocols may vary from initial protocols and/or some products may be required to sit in the lines overnight as compared to others with a five-minute time frame.
- Not all products are treated the same: Some products are used to clean (often referred to as “shock”) on a periodic basis, whereas others are meant to be used for ongoing maintenance of DUWLs (often daily or weekly) in conjunction with a cleaner. Treating cleaned waterlines to minimize subsequent microbial colonization may involve the same or a different product. For example, combination cleaner/maintenance system products are available which contain separate waterline cleaning (i.e. “shock”) agents and maintenance chemicals. They are very effective when used appropriately. This is where compliance with a manufacturer’s instructions for use procedures is important. In this instance, manufacturers include both a separate cleaner for periodic use to remove accumulated microbial contaminants, along with a maintenance product for more frequent, routine use. Others, especially those treatments employing the daily application of antimicrobial tablets, call for a separate cleaning solution. It should be noted using bleach as a DUWL cleaner and treatment is not approved by the EPA for dental waterline use. Corrosion and compromise of waterline components can occur with prolonged use of bleach. Multiple highly effective and compatible waterline cleaners have been extensively tested and are readily available with EPA approval for DUWL use.
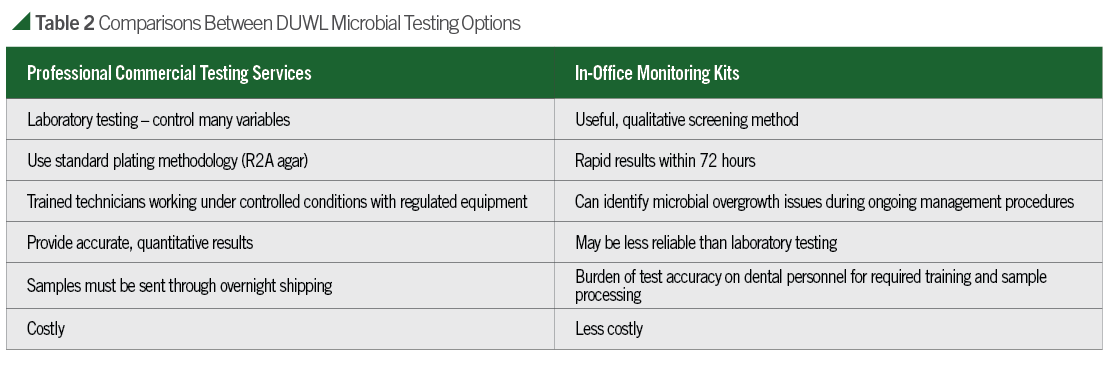
- Periodic DUWL testing: Periodic sampling and testing is the most reliable way to evaluate dental water quality. By doing this, it is possible to monitor compliance with IFUs and correct problems which may arise. Recently, the Organization for Safety, Asepsis, and Prevention (OSAP) published a white paper discussing infection control issues along with recommendations for maintaining dental water quality.8 In addition, The Centers for Disease Control and Prevention (CDC), American Dental Association (ADA), and other public health agencies recommend dental treatment water meets the EPA drinking water standard of less than 500 colony forming units per milliliter (CFU/mL). OSAP and an increasing number of state health departments have also recommended quarterly (or more often) testing of waterlines. Two available dental water quality testing options are mail-in water-testing services and in-office methods. Comparisons of both options can be found in Table 2. Whichever test method is used, it should be able to inform the user of an estimated count of colony forming units per milliliter (CFU/mL). When used appropriately and according to the instructions, monitoring products and services can aid dental facilities in accomplishing dental water quality goals.
Advances in microbial identification techniques and epidemiological investigations of waterborne outbreaks have allowed improvement in documentation of infections caused by a variety of bacteria. While it is difficult to eliminate waterborne pathogens as natural inhabitants in healthcare water systems, infections caused by these microorganisms are preventable.5
Multiple choices are available for controlling and monitoring colonization of water delivery systems and reservoirs, and other technologies continue to be developed. With specific regard to dentistry, the keys for accomplishing DUWL asepsis goals remain the same as for other infection control areas: One, application of basic infection prevention principles; and Two, compliance with use of approved products.
Colonized waterlines should be cleaned first to remove accumulated microbial and extracellular material prior to implementation of routine treatment procedures. Subsequent compliance with step-by-step IFUs is essential for controlling and maintaining low microbial levels in potable (<500 CFU/mL) treatment water. Periodic testing of water quality can also ensure that ongoing water treatment procedures are effective.
When monitoring is performed as a component of a practice’s DUWL infection prevention routine, it can assist in identifying compliance problems and also provide documentation of water quality.
References
1. Ricci MI, Fontana S, Pinci F, et al. Pneumonia associated with a dental unit waterline. Lancet 2012;379:684
2. Peralta G, Tobin-D’Angelo M, Parham A, et al. Notes from the field: Mycobacterium abscessus infections among patients of a pediatric dentistry practice – Georgia, 2015. Morbid Mortal Wkly Rpt 2016;65:355-356
3. Hatzenbuehler LA, Tobin-D’Angelo M, Drenzek C, et al. Pediatric dental clinic-associated outbreak of Mycobacterium abscessus infection. J Pediat Dent 2017;6:e116-122
4. Schonning C, Jernberg C, Klingenberg D, et al. Legionellosis acquired through a dent unit: a case study. J Hosp Infect 2017;96:89-92
5. Kanamori H, Weber DJ, Rutala WA. Healthcare outbreaks associated with a water reservoir and infection prevention strategies. Clin Infect Dis 2016;62:1423-1435
6. Molinari JA. The ongoing challenge of waterborne infections. Inside Dent. August 2017;52-58
7. Molinari JA. Dental waterline infection control: a work in progress. Dent Econ. February 2017;2
8. Mills SE, Porteous N, Zawada J. Dental unit water quality: Organization for Safety, Asepsis, and Prevention White Paper Recommendations – 2018. J Dent Infect Control Safety 2018;1:1-27

ACTIVA BioACTIVE Bulk Flow Marks Pulpdent’s First Major Product Release in 4 Years
December 12th 2024Next-generation bulk-fill dental restorative raises the standard of care for bulk-fill procedures by providing natural remineralization support, while also overcoming current bulk-fill limitations.