Align announces FDA clearance of iTero Element 5D Imaging System
Align Technology has received FDA 510(k) clearance for its iTero Element 5D Imaging System for commercial availability in the U.S.
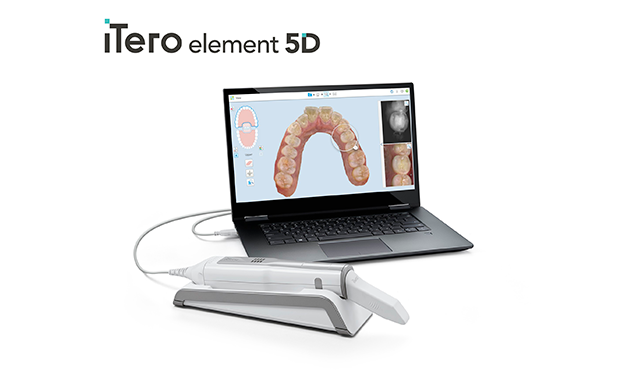
Align Technology has received FDA 510(k) clearance for its iTero Element 5D Imaging System for commercial availability in the U.S., the company announced this week.
The iTero Element 5D system expands the suite of existing high-precision, full-color, imaging, and fast scan times of the iTero intraoral scanner portfolio with a new clinical approach, optimized orthodontic and restorative dental workflows, and an improved doctor experience. In addition, iTero Element 5D’s visualization tools improve patient engagement and communication by helping patients see and better understand their current dental health, treatment plan options, and prescribed outcome on the iTero touchscreen. This is designed to enable better patient education, engagement, and compliance during treatment.
“The iTero Element 5D Imaging System seamlessly combines three key scanning technologies (3D data, intra-oral color photos and NIRI images) into one, integrated scan, and we are excited to bring this advancement in intraoral scanner technology to the U.S. market to help doctors provide better oral care for their patients,” said Yuval Shaked, senior vice president and managing director, Align Technology. “At the same time, we are mindful of the current environment and the impact that the COVID-19 pandemic is having across the world, and are focused solely on customer education regarding this new technology while so many dental practices in the U.S. are operating on a limited schedule.”
The iTero Element 5D scanner is said to be the first integrated dental imaging system that simultaneously records 3D, intraoral color, and NIRI images and enables comparison over time using the iTero TimeLapse function. This function aids doctors in detecting and monitoring the progression of interproximal caries above the gingiva without using radiation.
The iTero Element 5D is designed to complete a full arch scan in as little as 60 seconds and features analysis instruments, such as the Occlusogram occlusal clearance tool, and the Invisalign® Outcome Simulator and Progress Assessment.
The iTero Element 5D Imaging System will be commercially available in the U.S. in the second quarter of 2020 and is currently available in Canada, Latin America, European Union countries, the UK, Australia, New Zealand, Hong Kong, Thailand, Japan, Korea, Singapore, and Taiwan.
More information about the iTero system can be found at itero.com.
Oral Health Pavilion at HLTH 2024 Highlighted Links Between Dental and General Health
November 4th 2024At HLTH 2024, CareQuest, Colgate-Palmolive, Henry Schein, and PDS Health launched an Oral Health Pavilion to showcase how integrating oral and general health can improve patient outcomes and reduce costs.
Episode 31: Dentsply Sirona Implant Announcements
September 30th 2021DPR’s Editorial Director Noah Levine sat down with Gene Dorff, Dentsply Sirona’s group vice president of implants and Dr. Dan Butterman to review several big announcements the company made in the arena of implants during Dentsply Sirona World 2021 in Las Vegas.