How to go beyond conventional composites in restorative dentistry
How ORMOCER dental composites, such as the Admira Fusion family, can lead to clinical performance improvements.
Recent advancements in the evolution of light-cured dental composite restoratives include modification of flow, condensability, increased depth of cure, bulk placement, simplified finishing and polishing.
Filler particle changes, as well as minor chemistry modifications with varied acrylic resin mixtures, have contributed to these advancements. With each new handling feature manufacturers promote simplified clinical procedures, streamlined workflow and time savings. Data linking these advancements to clinical performance improvements however, are lacking.
While the clinical service life of direct placed dental composite restorations has increased over the past decade, additional improvements are still needed. More than 122 million dental composites are placed annually in the U.S. and fail, on average, within eight years, only to be replaced with another composite restoration. Research has attributed dental composite failure to degradation, stimulation of cariogenic micro-organisms by leachable resin components and biocompatibility issues.1-4 Citing various approaches to improving dental composites, in 2013 the National Institute for Dental and Craniofacial Research (NIDCR) announced funding to create the next generation dental composite. In the words of NIDCR director Dr. Martha Soloman, “The time is right scientifically to develop the next generation dental composite” focusing on the development of novel matrix resins resistant to hydrolytic degradation, improved biocompatibility, carioinhibition and microbial inhibition.1 With this announcement the search for a superior light-cured direct restorative had begun.
Related reading: VOCO releases Admira Fusion Flow
Drawbacks of methacrylate-based dental composites
Methacrylate (“acrylic”) resins made their dental debut as moldable, laboratory processed removable complete and partial dentures. Incorporation of amine activators in benzoyl peroxide (BPO) catalyzed monomethyl methacrylate (MMA)/polymethylmethacrylate (PMMA) mixtures and made intraoral auto-polymerization possible. PMMA powder, softened and partially solubilized by MMA liquid resulted in the world's first tooth colored direct acrylic resin restorations. Launched prior to classification of dental restoratives as medical devices, MMA+PMMA acrylic resin polymers had been regarded as safe despite recognition of the allergenic potential of MMA. In situ polymerization of these auto-polymerized methacrylate mixtures became common clinical practice before monomer volatility or leaching of un-reacted methacrylates were linked to biological risks and device longevity.
In 1976 the US Food and Drug Administration' classified dental restoratives as medical devices allowing all previous and current dental restoratives legally marketed in the US to be considered safe and effective unless ongoing marketplace surveillance suggested otherwise. This classification further allowed new products, including light-activated methacrylate-based resin mixtures, clearance to market based on manufacturer claims of substantial equivalency to devices marketed prior to 1976. Manufacturer claims of substantial equivalency, with ongoing product surveillance, opened the door for nearly all methacrylate-based resins to enter the U.S. market with a modicum of biocompatibility, toxicity and allergenicity testing.
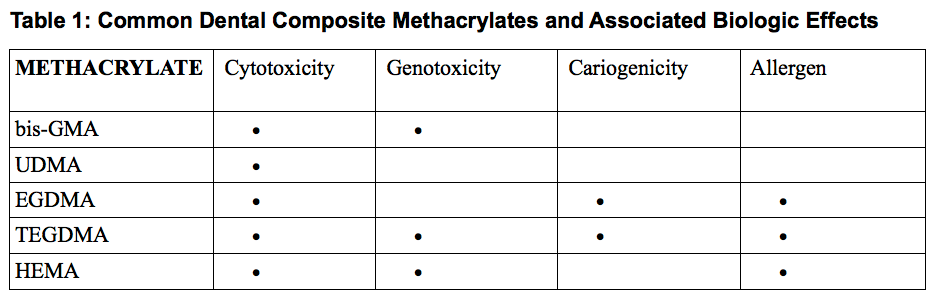
From bis-glycidyl dimethacrylate (bis-GMA), to urethane dimethacrylates (UDMA) to ethylene glycol dimethacrylates (EGDMA and TEGDMA) and hydroxyethylmethacrylate (HEMA), new “acrylics”, were added to dental composite formulations based primarily on carefully worded manufacturer claims of substantial equivalency with limited cell and animal testing. A quarter century after many substantially equivalent methacrylates entered the market; research began to suggest problems associated with cytotoxicity, genotoxicity including DNA disruption, allergy, endocrine disruption and stimulation of cariogenic microorganisms with reduced secondary dentin formation.5,6
The negative biologic effects of dental composite resin systems have been measured for specific methacrylate monomers, mixtures of methacrylates, as well as leached components from incompletely cured methacrylates with and without degradation products. Negative biologic effects cited above have been confirmed along with effects on oral biofilm and pulp vitality.7-9
General consensus exists that in vitro effects do not perfectly correlate with in vivo findings leaving room for speculation regarding real clinical outcomes. For instance, the level of influence for each effect depends on the specific chemistry of the dental composite, leaching medium, amount and type of extracted components, age of the restoration and degree of polymerization. Table 1 lists common methacrylates found in today's dental composites and reported in vitro and in vivo effects. It's important to note that while UDMA has been linked to the fewest biologic effects, many isomers of UDMA have been shown to cause significant effects. These isomeric differences explain some of the confusing research outcomes indicating that molecular configuration and purity can play an important role in biologic effects.9 Purity of sourced acrylics, especially those blended empirically to create various dental composites, is an ongoing concern. One very recent example of risk mitigation by a major dental manufacturer and its chemical supplier resulted in the discontinuation of a light-activated restorative and prosthetic dental composites.10,11
Trending article: How to use VOCO's Admira Fusion Universal Light-Cured Nano-ORMOCER
Continue to page two to learn about the future of dental composites...
The future of dental composites
With the need for a new generation dental composite clearly acknowledged in the U.S., similar concerns among European researchers had already spurred investigations into novel biocompatible dental polymers. Organically modified ceramics, ORMOCERS®, developed at Fraunhofer-Gesellschaft, Europe's largest organization for applied research showed promise. VOCO, the first dental manufacturer to successfully incorporate ORMOCER® chemistry into a resin dental composite, had already launched Admira, an ORMOCER® -based, light-cure direct restorative well ahead of the NIDCR announcement. Although Admira’s ORMOCER®-resin combination reduced the acrylic resin content resulting in a more robust restorative material, not all clinical issues were fully addressed. In 2016 VOCO launched dentistry's first all ceramic-based, pure ORMOCER® restoratives branded as Admira Fusion. The pure silicate matrix technology of Admira Fusion combined with nano-hybrid fillers resulted in dentistry's first “nano-ORMOCER®”. Today the Admira Fusion brand covers pure ORMOCER® restoratives available in both condensable and flowable viscosities as well as a universal shade, condensable, bulk-fill formulation. ORMOCER® technology truly represents today's next generation restorative replacing all methacrylate backbone resins as well as the methacrylate-based viscosity reducers, cross linking agents and hydrophilic acrylics linked to today's composite failures.
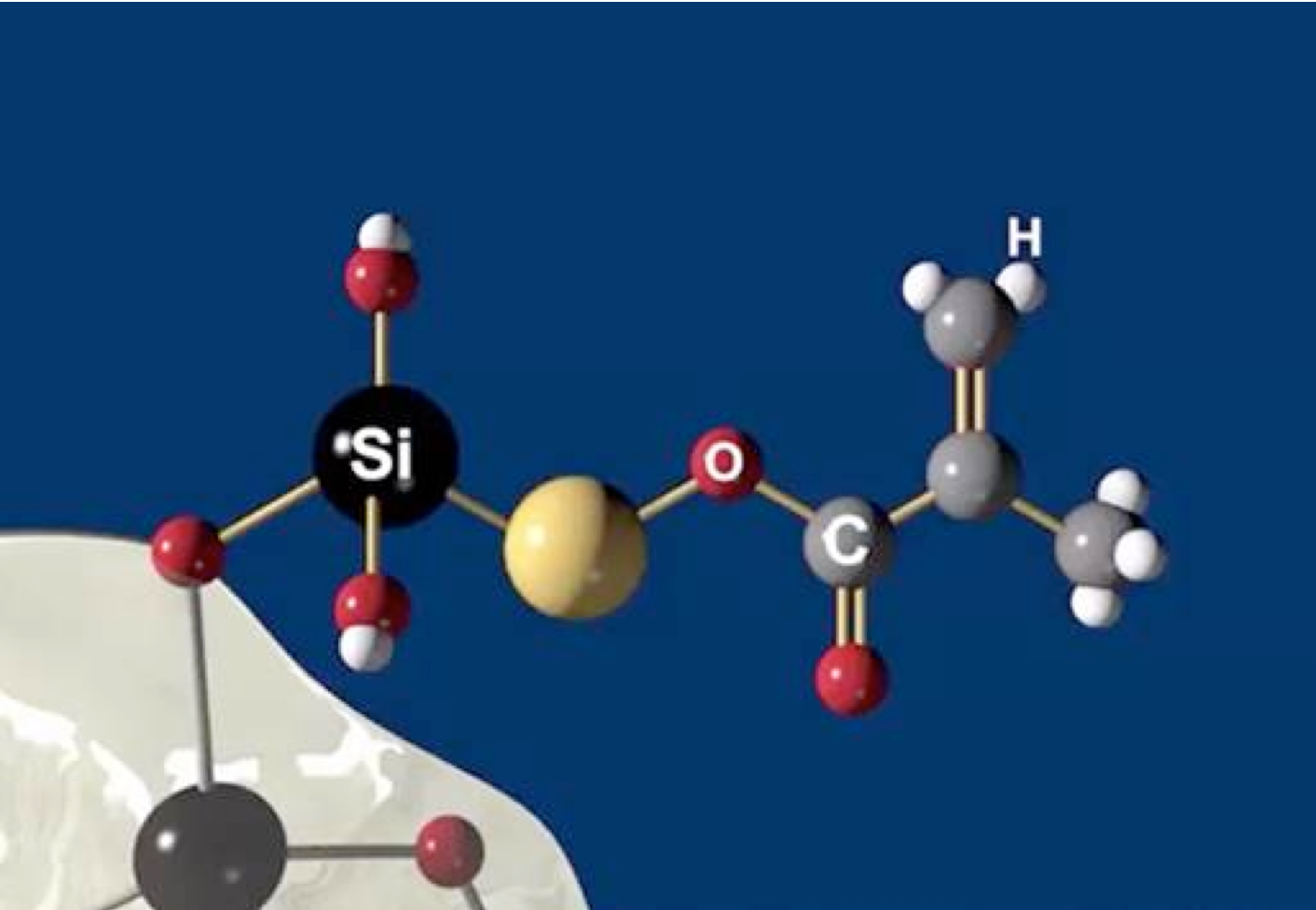
With no classic methacrylate monomers, Admira Fusion’s matrix consists of large and pre-condensed inorganic polymer molecules which readily photo-polymerize with significantly reduced shrinkage. Reduced polymerization shrinkage equates to reduced volume loss during curing. This conservation of volume minimizes stresses at bonded interfaces minimizing microgap formation and microleakage problems enhancing restoration integrity. While Admira Bond, chemically matched to bond Admira Fusion products, minimizes unwanted effects of methacrylate resins, it is not an essential component of the Admira Fusion line. Conventional enamel and dentin bonding agents may be substituted for Admira Bond without compromising bond strength. In addition, all Admira Fusion products are compatible with all base and liner formulations currently used with resin composites, glass ionomer, resin-modified glass ionomer, calcium hydroxide, mineral trioxide aggregate and calcium silicate formulations.
Trending article: Easy-to-use VOCO materials allow for natural-looking esthetics
One final comment related to dentistry's shift from organic to inorganic polymers relates to endocrine disruption effects of acrylic resins.8,13 Environmental accumulation of resins used in dentistry as well as in food and drink storage is increasing. Environmental hormone effects initially attributed to bisphenol A (BPA) are now known to involve other methacrylates. The pharmacokinetics and effects of BPA residues leached from bis-GMA dental resins have been reported although BPA quantities from dental resins are miniscule compared to quantities from food packaging. Less is known regarding endocrine disruption caused by common dental composite degradation products although there's clearly cause for concern. Certainly the risks associated with hormonal disruption potential of in dwelling medical devices as well as plastics in our environment warrant additional research. Until dental composite formulations and bonding resins are shown to leach zero BPA, patient concerns will continue.
Continue to page three for a clinical case...
Admira Fusion family: Clinical cases
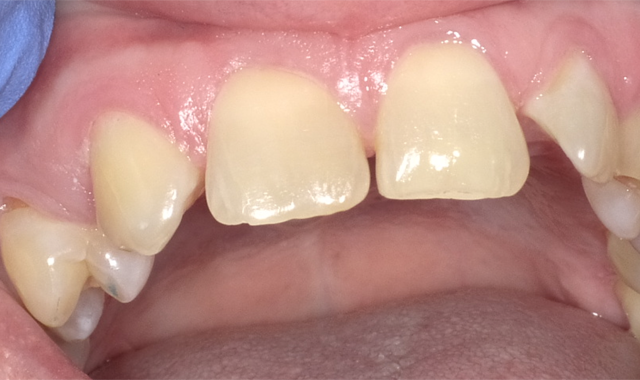
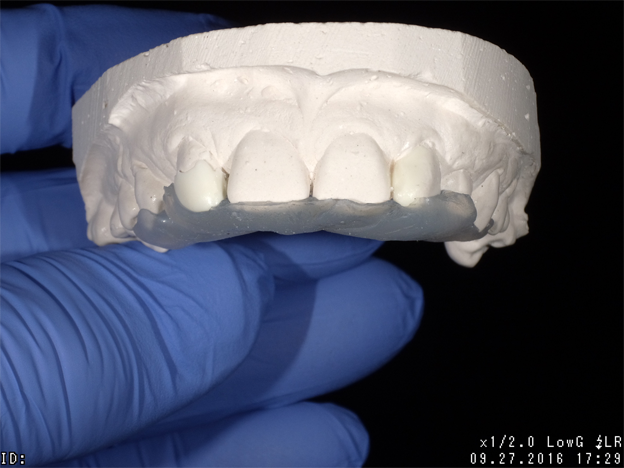
Admira Fusion restorative material, available in 17 shades, is indicated for all Class I-V dental restorations. Handling like a packable composite resin, Admira Fusion condenses easily into cavity preparation with light pressure providing good shape stability prior to curing. Figure 1 illustrates pre-treatment view of diastemata. A diagnostic cast was quickly constructed from an alginate impression and a silicone stent formed from a diagnostic wax-up (Fig. 2).
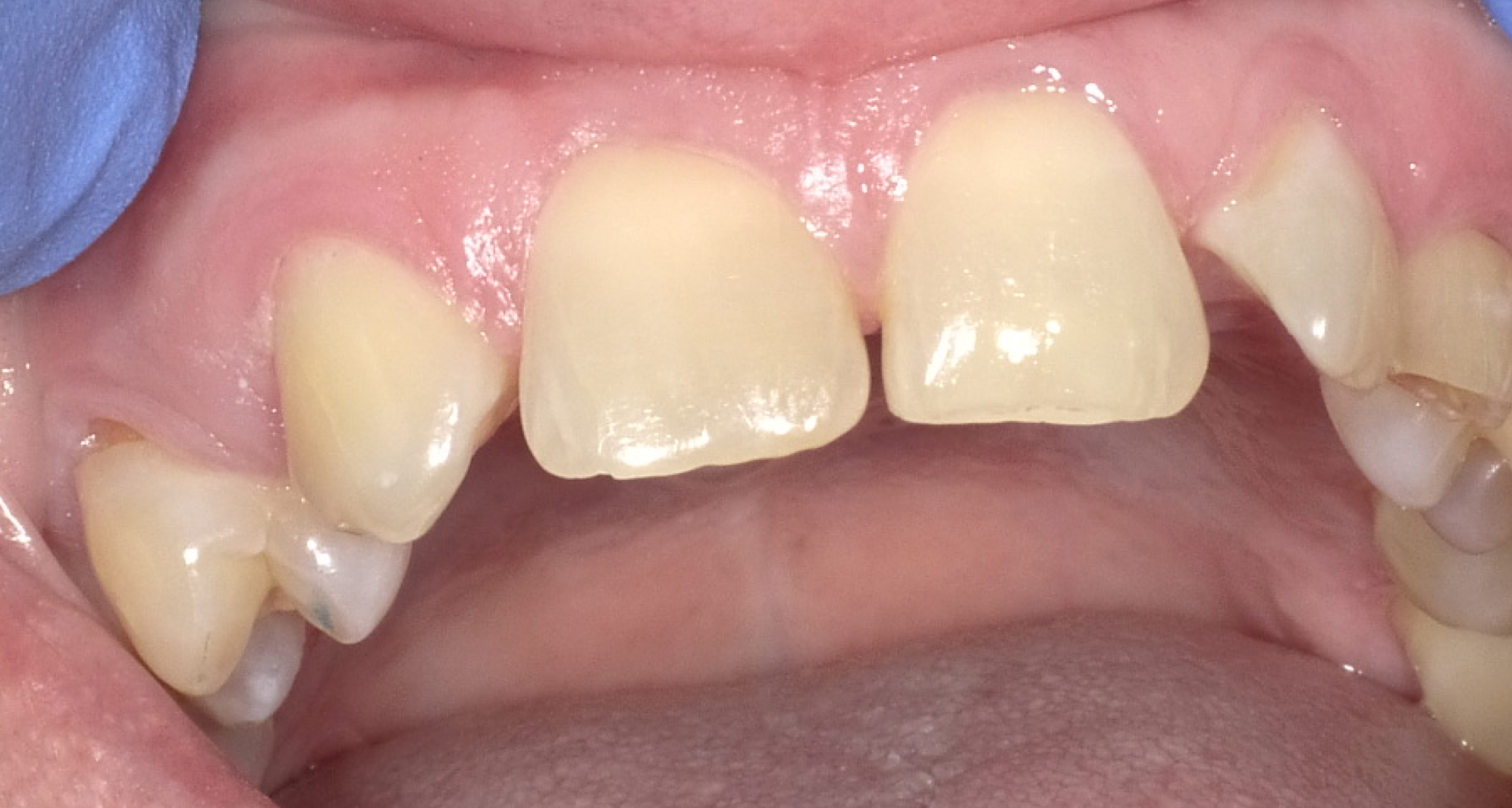
Using the silicone stent with Teflon tape, acid etching was completed with no rotary instrumentation of enamel (Fig. 3). Admira Fusion Flow incisal shade was placed (Fig. 4) and cured (Fig. 5), developing an outline form to resemble a lateral incisor.
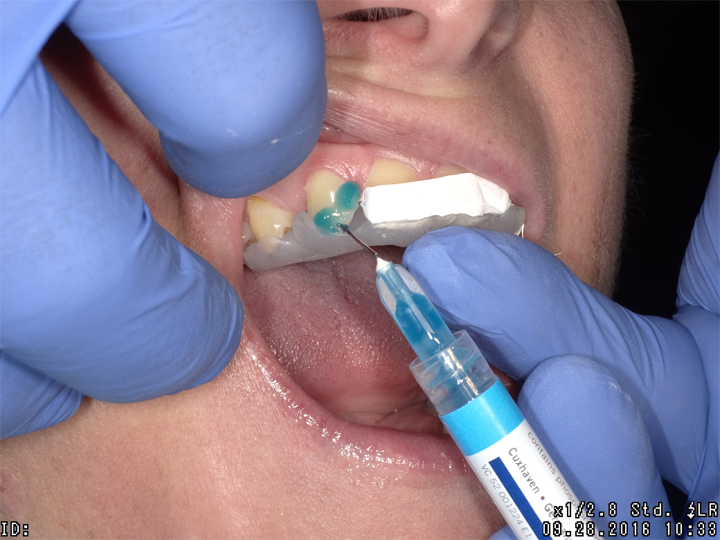
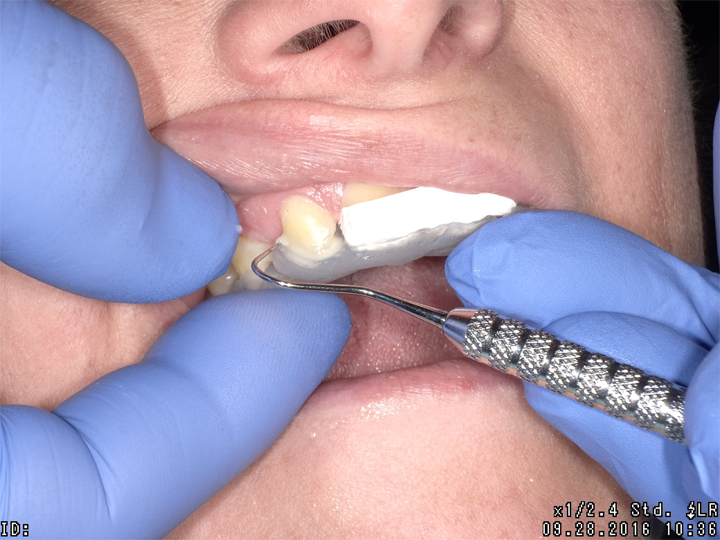
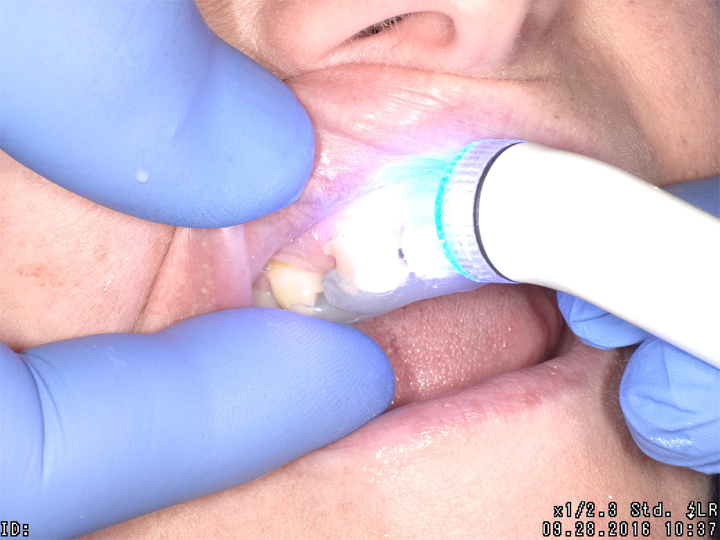
Fig. 3 Fig. 4 Fig. 5
Figure 6 shows the anterior re-contouring immediately after finishing and after 2 weeks (Fig. 7).
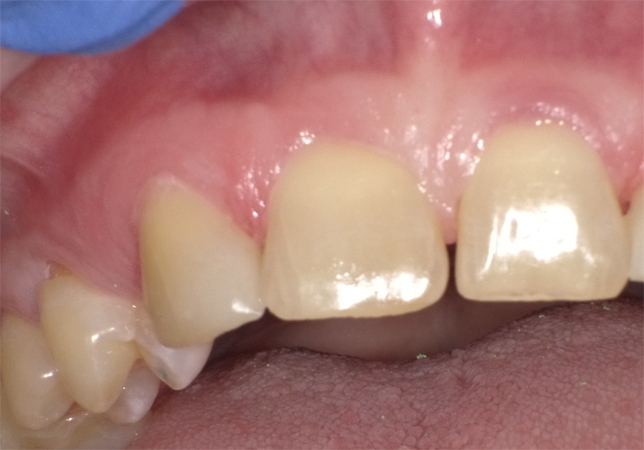
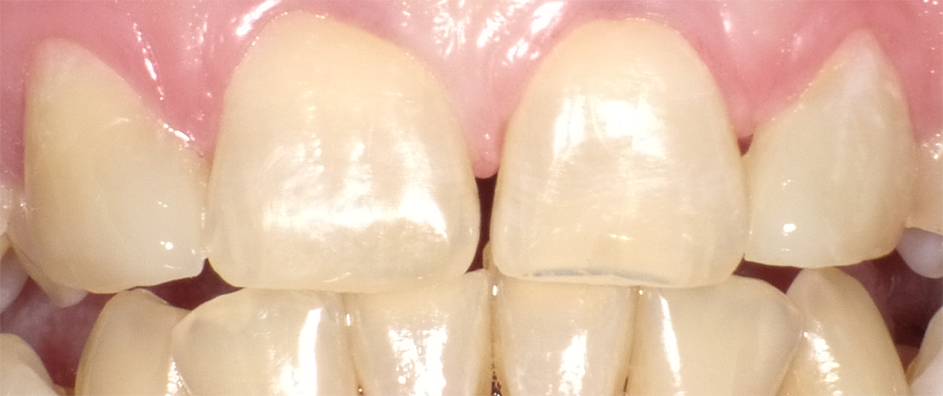
Continue to page three for more...
The next clinical sequence features Admira Fusion clinical steps restoring occlusal tooth #14 (Fig. 8). Tooth restoration was completed with a calcium hydroxide liner placed where remaining dentin thickness appeared to be 2 mm or less. Figure 9 illustrates completed cavity preparation, followed by placement of Admira Bond after 15 second phosphoric acid etching (Fig. 10).
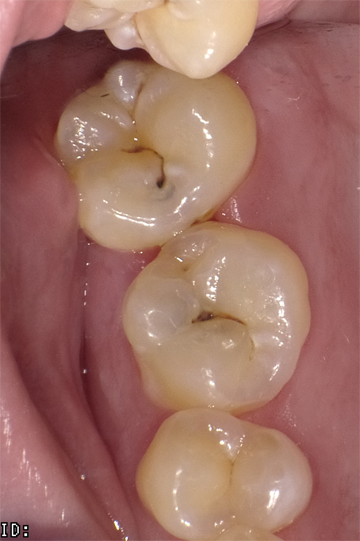
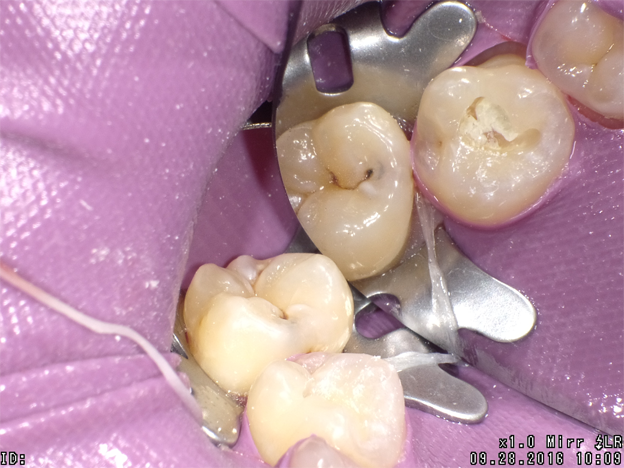
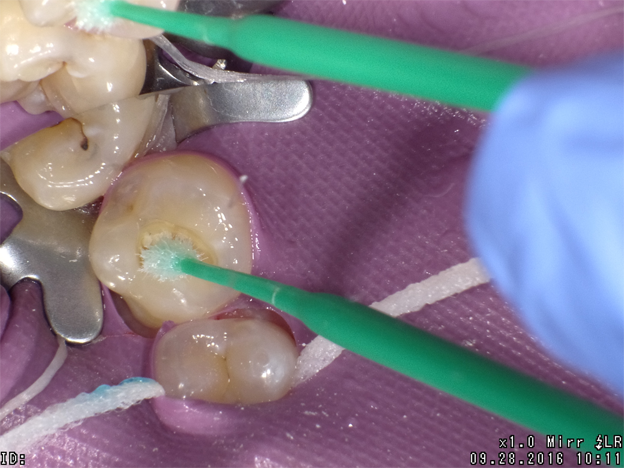
A 2 mm increment of Shade A3 which was then condensed and cured for 20 seconds with the DenMat LumiLight and veneered with a 2 mm incisal layer and cured for 20 seconds (Fig. 11). The cured restoration finishes and polishes easily using 6-fluted carbide finishing burs and abrasive cup shaped polishers due to the nano-hybrid fillers (Fig. 12).
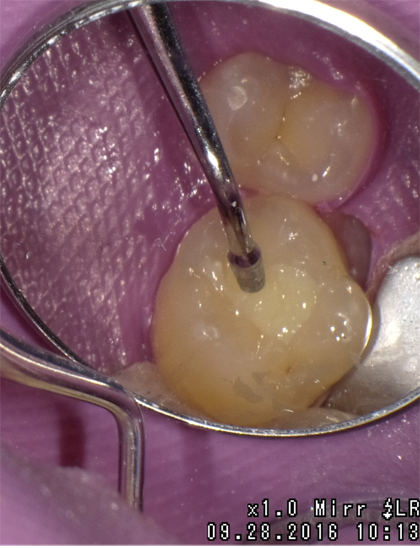
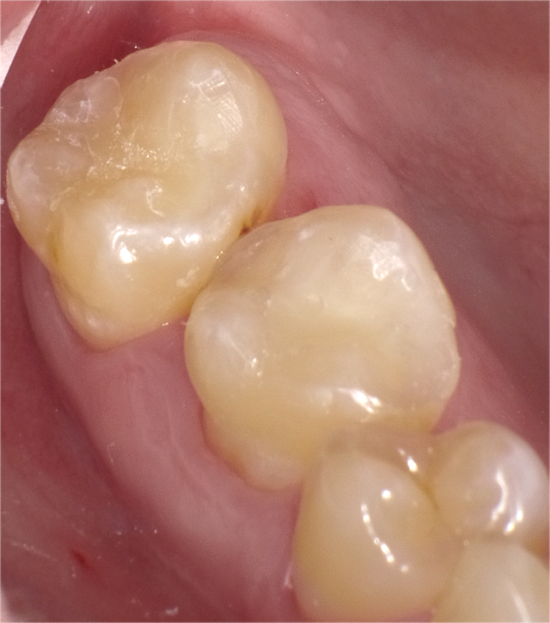
Fig. 11 Fig. 12
Occlusal restoration of the centralateral molar, tooth #3, is shown immediately after placement and curing (Fig.13). While tooth #14 was restored incrementally using a layer of Admira Fusion A2 veneered with Admira Fusion Incisal, tooth #3 was restored with Universal shade Admira Fusion x-tra, bulk-fill technique (Fig. 14). Polymerization shrinkage is exceptionally low for both Admira Fusion placed incrementally and is slightly higher when Admira Fusion x-tra is placed and cured in bulk, up to 4 mm thickness. Because of the esthetic limitations associated with a single shade, Admira Fusion x-tra is not indicated for Class III and Class IV (anterior) restorations, however the chameleon shading effects of a single shade material rivaled the esthetics of the incrementally placed, dual shaded restoration.
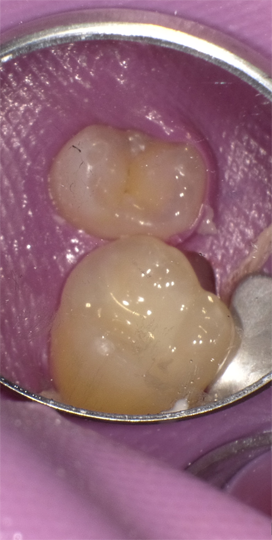
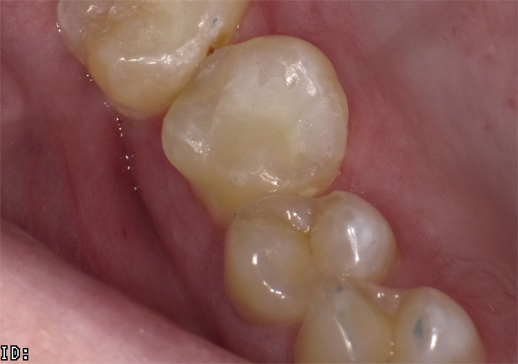
Fig. 13 Fig. 14
Admira Fusion is the world's first purely ceramic-based restorative containing nano-hybrid and glass ceramic fillers with a matrix resin backbone of silicon dioxide. Admira Fusion restoratives' inorganic polymer chemistry reduces polymerization shrinkage and shrinkage stress offering unprecedented biocompatibility. Admira Fusion's high edge strength, low water sorption, degradation resistance and extremely low solubility, equals or exceeds properties of today's leading dental composites.
Continue to page four for more...
Breakthrough product chemistry often requires clinicians to change clinical protocols or learn new techniques. The restoratives in the Admira Fusion product line offer clinicians the flexibility to employ the same tooth preparation, priming and bonding sequences used with today's bulk cure or incrementally placed dental composites
The anterior esthetic contour changes featured here were completed using Admira Fusion Flow after etching with phosphoric acid for 15 seconds before Futurabond U was applied, air-thinned and light-cured. Each bonded restoration was shaped with the aid of a transparent vinylpolysiloxane stent made on a corrected diagnostic cast. The outline form of #7 was created using Admira Fusion Flow Incisal shade followed by shade A2 Admira Fusion Flow formed using a mylar strip, then light-cured. At the restorative appointment, an alginate impression was used to create a Snap Stone model. Waxed-up tooth forms, and silicone stent were then constructed. Etched tooth surface received Admira Fusion Incisal followed by shade A2 Admira Fusion Flow, shaped using a mylar strip. Contouring was completed with an 8-fluted T6 finishing bur followed by polishing with a Patterson Green abrasive cup, a Patterson Yellow abrasive cup and a Caulk Enhance polishing cup.
Fig. 15
Dental dam isolation was used for posterior restorations placed after phosphoric acid etching for 15 seconds followed by air-water syringe rinsing, light air drying before applying Admira Bond with a microbrush. Admira Bond was then air-thinned and light-cured for 20 seconds. Admira Fusion and Admira Fusion x-tra were each introduced into their respective cavity preparation and condensed. Incremental curing was optional based on depth of the 4 mm depth of the cavity restored with Admira Fusion x-tra Universal Shade Restorations were completed and rotary contoured with an egg-shaped, 6-fluted finishing bur with water spray then polished with a Patterson Green abrasive polishing cup. The chameleon properties of pure silicate technology ORMOCERS® surpass those of earlier ORMOCERS® because of superior matching of the refractive indices of the polymer and filler particles. Superior translucency offers clinicians the advantage of a universal shaded dental composite which takes on the color of its surroundings, rivaling the appearance of layered restorations layered with A2 and incisal shades.
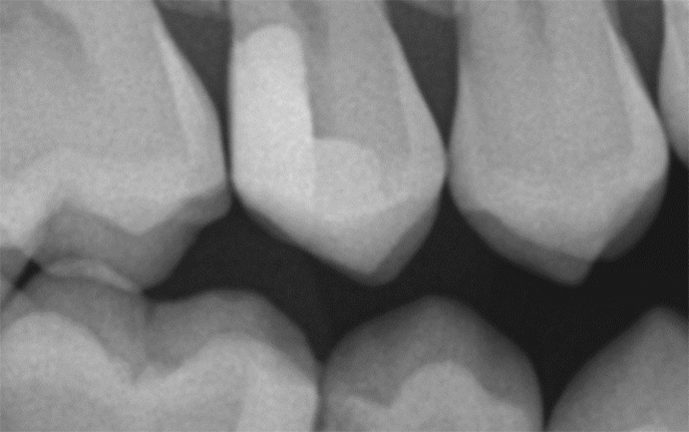
The all ceramic-based chemistry and high filler loading of Admira Fusion products combine to create a very radiopaque restorative material. Premarket evaluation comments specifically addressed Admira Fusion's remarkable radiodensity and superb esthetics, an uncommon combination.14Figure 15 illustrates a two surface Admira Fusion x-tra restoration at recall. The bitewing radiograph taken several months after restoration placement confirms radiopacity slightly exceeding that of enamel, a nice feature for esthetic restorative materials. It was known at the time of placement that advanced caries encroached on the dental pulp hence a thin calcium hydroxide liner was placed to protect the pulp and a dentin bridge is now evident. The patient reported 'no post-operative sensitivity' and no pulp problems have been identified.
Today dental clinicians have a myriad of esthetic dental composites restorative systems available to them. Systems offering a variety of shades, chromacities, translucencies, stiffnesses, flow, sculptability and other handling advantages fill dental journals, each seeking their niche in a crowded marketplace. Manufacturer claims related to slightly increased filler loading, minor filler particle modifications to create superior luster, easier placement, faster curing, simplified finishing, better esthetics and reduced shrinkage are the results of substantial equivalency thinking, raising the bar minimally. While incremental improvements are not without merit, substantially different chemistry is what's required when major improvements are needed. ORMOCER® “Pure Silicate Technology” represents a complete change in dental composite chemistry distinguishing Admira Fusion restoratives from the substantially equivalent crowd.
Continue to page five for references...
References
1. Kuska B, National Institutes of Health (September 5, 2013). NIH Funds Six Grants to Build Next Generation Dental Composite. Retrieved from https://www.nih.gov/news- events/news- releases/.
2. Van Landuyt KL, Nawrot T, Geebelen B, DeMunck J, Snauwaert J, Yoshihara K, Goddens L, Hoet P, Van Meerbeek B. (2011). How much do resin-based dental materials release? A meta- analytical approach. Dent Mater, 27(8): 723-47.
3.Bourba M, Ma D, Cvtikovitch DG, Santerre JP, Finer Y. (2013). Cariogenic bacteria degrade dental resin composites and adhesives. J Dent Res, 92(11): 989-94.
4.Spencer P, Ye Q, Misra A, Goncalves SE. (2014). Proteins, pathogens, and failure at the composite-tooth interface. J Dent Res, 93(12):1243-9.
5.Krifka S, Seidenader C, Hiler KA, Schmalz G, Schweikl H. (2012). Oxidative stress and cytotoxicity generated by dental composites in human pulp cells, Clin Oral Investig, 16(1):215-24.
6. Galler KM, Schweikl H, Hiler KA, Cavender AC, Bolay C, D'Souza RN, Schmalz G. (2011), TEGDMA reduces mineralization in dental pulp cells. J Dent Res, 92(2):257-62.
7. Urcan E, Scherthan H. Styllou M, Haertel U, Hickel R, Reichl FX. (2010). Induction of DNA double-strand breaks in primary gingival fibroblasts by exposure to dental resin composites. Biomaterials, 31(8):2010-14.
8. Goldberg M. (2014). BPA from dental resin material:where are we and where are we going with restorative and preventive dental biomaterials. Clin Oral Investig, 18(2):347-9
9. Invitro and in vivo studies on the toxicity of dental resin components: a review. Clin Oral Investig, 12(1):1-8.
10. Polydorou O, Konig A, Hellwig E, Kummerer KJ. (2009). Urethane dimethacrylate: a molecule that may cause confusion in dental research. Biomed Mater Res Appl Biomater, 91(1):1-4.
11. Written communication. (September 14, 2016). K. Bonser, Dentsply Prosthetics.
12. Telephone conversation. (October 4, 2016). Senior Research Chemist, Dentsply Prosthetics R&D.
13 . Goldberg M, Dimitrova-Nakov S, Schmalz G. (2014). Cytotoxicity of dental composites and their leached components. Clin Oral Investig, 18(2):347-9.
14. The Dental Advisor https://www.dentaladvisor.com/evaluations/admira-fusion-x-tra/
ACTIVA BioACTIVE Bulk Flow Marks Pulpdent’s First Major Product Release in 4 Years
December 12th 2024Next-generation bulk-fill dental restorative raises the standard of care for bulk-fill procedures by providing natural remineralization support, while also overcoming current bulk-fill limitations.