Case study: Synthesis and application of a novel pre-polymerized filler for dental materials
A look at the new generation of TPH Spectra, TPH Spectra ST with SphereTEC filler technology.
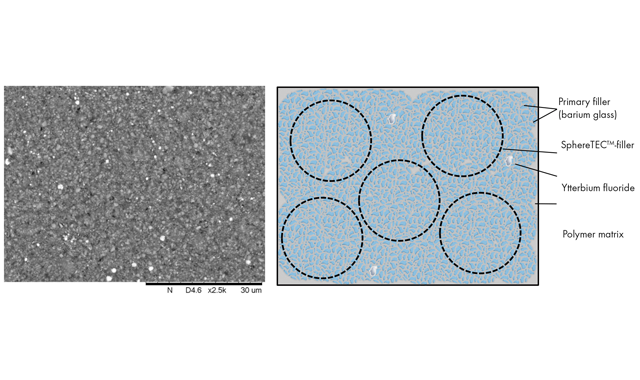
The composition of Commercially available, resin-based composites has significantly evolved over the last 60 years. While basic ingredients – essentially particular fillers, polymerizable resin, initiators, stabilizers and pigments – remain the same, numerous improvements in design and arrangement of components as well as mode of curing have been achieved.
Fillers constitute the major portion of a dental composite. Their chemical composition, morphology, surface condition and modification, average particle size as well as particle size distribution determine a significant part of the mechanical, esthetic and handling properties of the final material. Depending on their average size, corresponding composites are commonly categorized into macrofills (10-50 μm), midifills (1-10 μm), minifills (0.6-1 μm), nanofills (5-100 nm) or composites with mixtures thereof, so called hybrids.1 Furthermore, modern hybrids frequently contain pre-polymerized fillers (PPF). PPF are usually made from ground particles of either mini-/nanofill-composite or inorganic clusters and have a relatively low surface area compared to the sum of included, isolated particles.
Read more: TPH Spectra composite: 3 tips for shade matching
We report the synthesis and application of a novel type of size-, shape- and microstructure controlled pre-polymerized filler for dental materials. The PPF consists of defined superstructures of barium glass, bound with polymerized resin. The synthesis, titled SphereTEC™ 2, comprises three steps (Fig. 1).
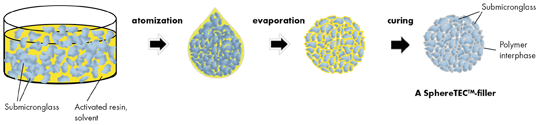
Fig. 1
First, a suspension of submicron barium glass in a mixture of solvent and resin is forced through a nozzle and into a hot air stream (atomization). When entering the gaseous phase, the emerging droplets immediately act to minimize their surface energy and form spherical shapes of a distinct size distribution. Due to the increased temperature, the solvent is subsequently evaporated and the initial droplets solidify into preformed particles. Finally, the resin on the particles’ interfaces is thermally cured and the PPF can be collected. Because of the well-controlled processing, fillers from this process can be used as obtained - additional milling is not required. Differently to most PPF, SphereTEC™-fillers are consequently characterized by a high degree of sphericity and distinct microstructure (Fig. 2).
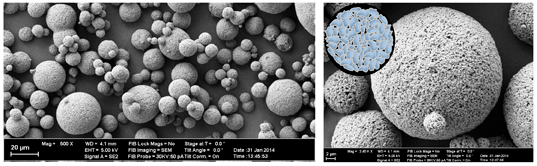
Fig. 2
By varying the process parameters, the average particle size can be altered from about 10 up to 70 μm. For the use in TPH Spectra® ST, an average size of 15 μm was found to be ideal. According to mercury intrusion, the intra-particle volume of these fillers amounts to ~0.1 mL/g with an average network-arc length of ~0.2 μm. Because of the particles’ microstructure as well as polymer-covered surfaces, uncured methacrylate resin is able to fully infiltrate and cover the particles (Fig. 3). Physical laws regulating this process are capillary forces and matching polarities of the fillers’ surfaces and penetrating resin. Full penetration is important to achieve homogeneity and a reduced stickiness of TPH Spectra® ST paste as well as adequate strength of the cured material.
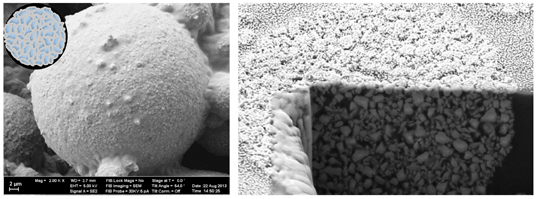
Fig. 3
For TPH Spectra® ST formulation, SphereTEC™-PPF and activated resin are combined with additional submicron glass. This is identical to the one used in the SphereTEC™-PPF and helps to further increase the packing density. Furthermore, a common radiopacifier, ytterbium fluoride, is added. Upon mixing, SphereTEC™-fillers fully blend into the overall composition, and are not distinguishable from other parts of the filler system (Fig. 4).
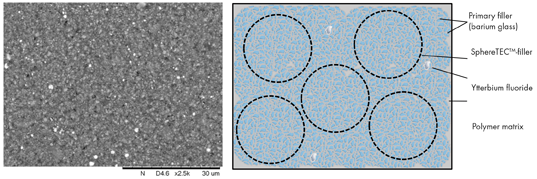
Fig. 4
Because of the carefully designed polymer interphases within the PPF, the composite’s susceptibility to wear is reduced, while the gloss corresponds to that of a highly filled microhybrid. When compared to using only isolated submicron glass, the total filler load is increased by more than 5 wt.-% to a maximum of 80 wt.-%.* Due to the uniform particle size of primary filler and matched refractive indices of inorganic portion to polymerized resin, optimal aesthetic properties with a high translucency of the material are achieved. The latter is a precondition for the color match of TPH Spectra® ST to the surrounding tooth-substrate, often titled as ‘chameleon-effect’. In addition to that, the special, convex surface of SphereTEC™-fillers positively influences the flow-properties of the paste. In combination with irregularly shaped primary fillers, distinct thixotropy results. This can be quantified by rheological measurements and depicted as the time-dependent ratio of viscous to elastic components tan δ = loss modulus G” storage modulus G’ (Fig. 5).
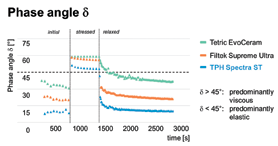
Fig. 5
As can be seen, TPH Spectra® ST exhibits low δ-values at low shear-stress, δ-values >45° at high shear stress and returns to low δ-values in the relaxed state. In terms of restoration therapy, this corresponds to an easy extrusion of TPH Spectra® ST from the Compule® Tips, good adaptation and sculptability (high δ-values, predominantly viscous properties) and a high slump resistance when left unagitated (low δ-values, predominantly elastic properties).
Trending research: Study finds virulent bacteria linked to caries in children
In conclusion, by providing a new technology, SphereTEC™, the production of spherical, pre-polymerized fillers of a defined size distribution and microstructure was achieved. Using these fillers in the new TPH Spectra® ST, advantageous properties of thixotropic rheology, reduced stickiness to hand instruments, appealing aesthetics with ‘chameleon effect,’ increased resistance to wear as well as adequate mechanical strength was achieved.
*The total filler load varies by shade from 76-80 wt.-% (57-62 vol-%), the corresponding inorganic filler content from 71-74 wt.-% (48-52 vol-%).
References
1. Ferracane, J. L., Resin composite--state of the art. Dental materials : official publication of the Academy of Dental Materials 2011, 27 (1), 29-38.
2. Stelzig, S.; Kempter, J.; Noerpel, S.; Klee, J. E.; Facher, A.; Walz, U.; Weber, C. Composite Filler Particles and Process for the Preparation thereof. WO2013/087223 A1, 2011.
ACTIVA BioACTIVE Bulk Flow Marks Pulpdent’s First Major Product Release in 4 Years
December 12th 2024Next-generation bulk-fill dental restorative raises the standard of care for bulk-fill procedures by providing natural remineralization support, while also overcoming current bulk-fill limitations.